Abstract
Background and aims
Probability discounting refers to the effect of outcome uncertainty on decision making. Using probability discounting, we examined the degree to which self-identified chronic pain patients (CPP) were likely to try a novel analgesic medication given increasing addiction risk. We postulated that propensity for opioid misuse, trait impulsivity, and previous opioid experience would be positively associated with likelihood of risky medication use.
Design
This cross-sectional online study determined state/trait associations with addiction-related medication decisions in CPP.
Setting
US-based CPP participated via Amazon Mechanical Turk; data were collected and analyzed in Baltimore, Maryland.
Participants
263 CPP (70% female) participated in the study from December 12–13, 2014.
Measurements
CPP responded to the Benefit vs. Addiction Risk Questionnaire (BARQ) assessing likelihood of taking a hypothetical once-daily oral analgesic medication as a function of two factors: risk of addiction (0%–50%) and duration of expected complete pain relief (3, 30, or 365 days). The primary outcome was the BARQ, quantified as area-under-the-curve (AUC). Grouping of CPP at high or low risk for opioid misuse was based on the Screener and Opioid Assessment for Patients in Pain-Revised (SOAPP-R). Predictors included previous experience with opioids, as well as various measures of chronic pain and mental health.
Findings
Across hypothetical addiction risk assessed in the BARQ, the likelihood of taking a novel analgesic medication was significantly elevated in patients with high (≥18; n=137) versus low (<18; n=126) SOAPP-R scores (p<0.001; 3-day: Cohen’s d = 0.66, 95% CI 0.63, 0.69; 30-day: d = 0.74, 95% CI 0.71, 0.78; 365-day: d = 0.75, 95% CI 0.72, 0.79).
Conclusions
In the USA, self-identified chronic pain patients (CPP) at higher risk for opioid misuse were more likely to report willingness to try a novel analgesic despite increasing addiction risk than CPP with low risk of opioid misuse.
Keywords: Addiction, Chronic pain, Analgesic medications, Probability discounting, Decision-making, BARQ, SOAPP-R
Introduction
As major national and international health organizations have advocated for the availability of opioid medications for the treatment of chronic pain, the use of these medications has risen – especially in the United States (1–3). Unfortunately, this increased use has been associated with the growing prevalence of opioid use disorder (OUD) and a steep rise in the incidence of opioid-related overdose deaths, with the number of annual overdose deaths overtaking motor vehicle accidents as the leading cause of accidental death in the United States (4). The coverage of these statistics in the popular press and online media has been dramatic, most likely reaching the majority of chronic pain patients and opioid prescribers (5, 6).
Given the aforementioned risks, regulatory bodies have advocated requiring informed consent conversations prior to initiating opioid medications for the treatment of chronic pain in an attempt to reduce overall opioid prescriptions and prevent harm (7). Part of this process involves an appraisal of the potential development of OUD (8), but the exact content of the conversation is left to the provider. However, communication about treatment between patients and providers is often equivocal (9). For example, a preliminary risk assessment of opioid misuse may vary greatly between providers if it is even addressed.
Little controlled research has examined how chronic pain patients (CPP) interpret conversations about addiction risk in determining when and if to initiate opioid therapy. The research surrounding CPP/provider interactions has focused primarily on other opioid side effects and satisfaction (10), discontinuation of opioids once started (11), and reasons why prescribers choose or do not choose to prescribe opioids (12, 13). Interestingly, chronic neuropathic pain patients identify the most important features of an analgesic medication to be pain relief, lack of nausea, and lack of character change (or unusual behaviors) (14), suggesting that chronic pain patients have preferences that include a consideration of medication risks and benefits. However, these preferences are often lost during standard brief patient/provider interactions, especially in emergency rooms 15,16.
There is a pressing need to quantify patient treatment preference, especially in a way that is generalizable to the chronic pain population and takes into consideration the benefits versus risks of a pharmacotherapy (15). Therefore, this manuscript presents results from an online survey of self-identified CPP who completed a novel probability discounting task wherein they were asked to weigh the benefits of various durations of pain relief against the risk of addiction resulting from once daily administration of a novel (and hypothetical) analgesic medication. Probability discounting is a behavioral process in which the uncertainty of an outcome affects decision-making, and is most often studied in the context of monetary choices in humans (16) e.g. choice between certainty in receiving a small reward versus varying probability in receiving a large reward (17). In the current study, the uncertainty of a negative outcome (developing addiction) was systematically manipulated and weighed against an increasing reward (duration of complete pain relief). The aims of this study were to (1) compare the likelihood of trying a novel analgesic between self-identified CPP with either high or low risk of opioid misuse, (2) determine the association between state/trait measurements (e.g. chronic pain, impulsivity) and probability discounting of addiction risk, and (3) determine whether previous opioid side-effects are associated with willingness to try a novel analgesic.
Methods
Design
In order address the aims of this study, we designed a survey that could be distributed online to reach a diverse sample of self-identified CPP. The survey included a probability-discounting task that was specific to the relationship between duration of pain relief versus the risk of addiction. In addition, CPP were asked to describe their pain and answer several standardized questionnaires assessing their risk for opioid misuse, state/trait measurements of mental health, and previous experience with prescription opioids (if any). The following sections characterize the patient population and describe the specific instruments utilized in the survey.
Participants
The study sample was composed of adults reporting chronic pain that resided in the United States, and registered as “workers” on the Amazon Mechanical Turk (AMT) platform. AMT has been used in numerous biomedical research studies and produces a nationally representative, unbiased sample with valid results (18–21). It is quicker and less expensive to recruit participants; e.g. this study sample was recruited in two days and each participant was paid between $1.50–3 for survey completion. Within the AMT platform, “human intelligence tasks” or HITs are made available to “workers” by “requestors” who rate whether the worker has completed the task to satisfaction. For the present survey, at least a 95% past approval rating for previous work on AMT was required to accept the HIT. Participation was voluntary and anonymous, and consent was indicated via completion of the main online survey. The Johns Hopkins University Institutional Review Board approved this study.
Our pre-specified desired number of completed surveys was 300, and, 647 participants filled out a brief screening survey to achieve this number of completed surveys. The screening survey assessed basic entry criteria for the study, including accurate answers on two distractor questions. Eligibility criteria were: (1) age ≥ 18; (2) resident of the United States; (3) presence of chronic pain. Pain was measured using a 5-point scale consisting of “none,” “mild,” “moderate,” “severe,” and “very severe”. Chronic pain was considered present if all of the following criteria were met: (a) having pain present for at least three months, (b) reporting past-week pain intensity of at least moderate at its worst, (c) reporting at least mild pain on average and (d) reporting that the intensity of the last pain experienced was ≥2 (using an 11-point visual analog scale). All surveys were hosted on Qualtrics (Provo, UT) and completed from December 12th–13th, 2014. A previously published manuscript from this sample reported on results from novel discounting tasks that assessed hypothetical pain-related rewards and punishments 22.
Measures
Benefit vs. Addiction Risk Questionnaire (BARQ)
The BARQ is a novel probability discounting task assessing one’s likelihood of taking a hypothetical analgesic medication given a duration of complete pain relief versus risk of developing addiction (Please see Supplemental Document 1 for exact instructions and instrument). Instructions prior to each set of questions asked the participant to imagine that a new analgesic medication has been approved for their pain condition, and that the medication is to be taken once daily by mouth. Instructions asked the participant to take questions seriously, to assume their current (personal) pain levels, and to weigh benefits (duration of complete pain relief in days) with risks (developing addiction). Addiction was not defined for participants. For the three day pain relief condition, participants were asked a series of nine questions regarding risk of addiction; addiction risks included the following (in order of presentation): 0%; 1/10,000 (.01%); 1/2,000 (.05%); 1/700 (0.14%); 1/400 (.25%); 1/100 (1%); 1/13 (8%); 1/3 (33%); and 1/2 (50%). After each proposition, participants were asked to gauge whether or not they would take the analgesic using a 0–100 visual analog scale (VAS) that had the following anchors: “Definitely not take” at 0 and “Definitely take” at 100. This sequence of questions was repeated for conditions proposing 30 and 365 days of pain relief. The percentages of addiction risk largely corresponded to prior probability discounting research in our unit that produced orderly results on a sexual probability discounting task (22). In addition, the BARQ probabilities encompass the range of addiction rates that have been reported for existing opioid analgesics (23, 24).
SOAPP-R
Participants completed the validated Screener and Opioid Assessment for Patients in Pain-Revised (SOAPP-R) (25). This 24-item scale assesses the frequency of risk factors that are predictive of misuse during an episode of opioid pain medication treatment. Though the SOAPP-R contains items that are face-valid for opioid misuse (“How often have you run out of pain medication early?”), it was intentionally designed to limit deception by patients (26). For example, the SOAPP-R also contains questions on the frequency of “tension in the home,” arguments resulting in physical violence, and impatience with doctors. For each risk factor, participants can answer “never”, “seldom”, “sometimes”, “often”, or “very often.” The items are summed for a total score (range 0–96). Patients with a total score of ≥18 have been shown prospectively to be at greater risk for opioid misuse during treatment (27). In the present study, total scores were calculated for the SOAPP-R, and a dichotomous variable for opioid misuse risk was created using established SOAPP-R cutoffs (≥18 or <18). Table 1 shows differences in participant characteristics broken down by SOAPP-R category; individuals scoring ≥18 are considered to be at heightened risk for opioid misuse (27).
Table 1.
Participant Characteristics.
| Total (N=263) |
Low SOAPP-R (N=126) |
High SOAPP-R (N=137) |
p-value | ||
|---|---|---|---|---|---|
| Age - Years (SD) | 46.9 (12) | 49.1 (12.7) | 44.9 (11.1) | 0.01 | |
| Sex (% Female) | 70.6 | 72.8 | 68.6 | 0.46 | |
| Sexual Orientation (% Non-heterosexual) | 11.8 | 5.6 | 17.5 | <0.01 | |
| Hispanic | 6.8 | 4.8 | 8.8 | 0.2 | |
| Race (% Caucasian) | 87 | 89.7 | 84.7 | 0.23 | |
| Education | High School Graduate | 7.6 | 7.1 | 8 | 0.09 |
| Some College | 48.7 | 42.9 | 54 | ||
| Bachelor’s Degree | 35 | 37.3 | 32.9 | ||
| Graduate Degree | 8.8 | 12.7 | 5.1 | ||
| Annual Household Income | $0–10,000 | 6.8 | 1.6 | 11.7 | 0.02 |
| $10,000–20,000 | 9.1 | 7.9 | 10.2 | ||
| $20,000–35,000 | 17.9 | 20.6 | 15.3 | ||
| $35,000–50,000 | 23.6 | 23.8 | 23.4 | ||
| $50,000–75,000 | 20.5 | 22.2 | 19 | ||
| $75,000–100,000 | 16.4 | 15.1 | 17.5 | ||
| $100,000–200,000 | 5.7 | 8.7 | 2.9 | ||
| Pain - Last Experienced | Today | 77.6 | 73.8 | 81 | 0.19 |
| Within Last Week | 18.6 | 22.2 | 15.3 | ||
| Within Last Month | 2.3 | 2.4 | 2.2 | ||
| 1–3 Months Ago | 0.8 | 0 | 1.5 | ||
| 4–6 Months Ago | 0 | 0 | 0 | ||
| >6 Months Ago | 0.8 | 1.6 | 0 | ||
| Pain - How Long Experienced | 3–6 Months | 4.6 | 4 | 5.1 | 0.65 |
| 6–12 Months | 10.3 | 11.9 | 8.8 | ||
| >12 Months | 85.2 | 84.1 | 86.1 | ||
| Pain – Frequency | At All Times | 17.9 | 17.5 | 18.3 | 0.69 |
| Daily | 49.4 | 46 | 52.6 | ||
| At Least 3× per Week | 24.3 | 26.2 | 22.6 | ||
| Once per Week | 3.8 | 5.6 | 2.2 | ||
| 3× per Month | 2.3 | 2.4 | 2.2 | ||
| 1× per Month | 1.9 | 1.6 | 2.2 | ||
| <1× per Month | 0.4 | 0.8 | 0 | ||
| Cause of Pain (Diagnosis with >10% respondents) | Arthritis | 37.3 | 39.7 | 35 | 0.44 |
| Neuropathy | 28.9 | 24.6 | 32.9 | 0.14 | |
| Other | 25.5 | 27.8 | 23.4 | 0.41 | |
| Headaches | 24.3 | 21.4 | 27 | 0.29 | |
| Disc Problems | 20.5 | 15.1 | 25.6 | 0.04 | |
| Knees | 20.2 | 19.1 | 21.2 | 0.67 | |
| Joints (Generally) | 20.2 | 19.1 | 21.2 | 0.67 | |
| Fibromyalgia | 13.7 | 16.7 | 11 | 0.18 | |
| Sciatica | 10.7 | 9.5 | 11.7 | 0.57 | |
| Total # of Pain Diagnoses (SD) | 2.7 (1.9) | 2.7 (2) | 2.7 (1.9) | 0.72 | |
| BPI - Primary Pain (SD) | Worst (0–10 VAS) | 6.5 (2.2) | 6.2 (2.3) | 6.7 (2.1) | 0.06 |
| Least (0–10 VAS) | 3.2 (2.4) | 3 (2.3) | 3.4 (2.5) | 0.2 | |
| Average (0–10 VAS) | 5.4 (1.6) | 5.2 (1.6) | 5.5 (1.7) | 0.06 | |
| Right Now (0–10 VAS) | 4.5 (2.5) | 4.2 (2.5) | 4.8 (2.4) | 0.07 | |
| Severity | 4.9 (1.8) | 4.7 (1.9) | 5.1 (1.8) | 0.048 | |
| BPI - % Relief from Treatments | 44 (29.5) | 46.6 (31.6) | 41.6 (27.4) | 0.17 | |
| BPI - Pain Interference | 5.1 (2.4) | 4.1 (2.3) | 6 (2.1) | <0.01 | |
| Exposure to Opioid Analgesics (% Yes) | 66.2 | 57.1 | 74.5 | <0.01 | |
| Duration of Exposure to Opioids | <1 Month | 27 | 38.9 | 18.6 | <0.01 |
| 1–3 Months | 17.8 | 8.3 | 24.5 | ||
| 4–6 Months | 10.3 | 9.7 | 10.8 | ||
| 7–9 Months | 4.6 | 8.3 | 2 | ||
| 10–12 Months | 5.8 | 6.9 | 4.9 | ||
| >12 Months | 34.5 | 27.8 | 39.2 | ||
| PHQ-2 (SD) | 1.9 (1.9) | 0.86 (1.2) | 2.8 (1.9) | <0.01 | |
| GAD-7 (SD) | 6.5 (5.7) | 3.09 (3.36) | 9.7 (5.6) | <0.01 | |
| PCS (SD) | Total | 23.2 (12.6) | 17.4 (10.9) | 28.5 (11.8) | <0.01 |
| Rumination | 8.4 (4.5) | 6.7 (4.3) | 10 (4.1) | <0.01 | |
| Magnification | 4.8 (3) | 3.5 (2.6) | 6 (2.9) | <0.01 | |
| Helplessness | 9.9 (6.1) | 7.2 (5.1) | 12.5 (5.9) | <0.01 | |
| ISI (SD) | Total | 11.9 (6.7) | 8.6 (6) | 14.8 (6) | <0.01 |
| Barrett Impulsiveness Scale, Version 11 (SD) | Attentional | 15 (4.4) | 12.9 (3.8) | 16.9 (4.1) | <0.01 |
| Motor | 20.3 (4.5) | 18.6 (3.5) | 21.8 (4.8) | <0.01 | |
| Non-planning | 23.1 (5.6) | 20.9 (4.5) | 25.1 (5.9) | <0.01 |
Values represent % of the group unless or mean if (SD) is specified. Abbreviations: Screener and Opioid Assessment for Patients in Pain-Revised (SOAPP-R); Visual Analogue Scale (VAS); Brief Pain Index (BPI); Patient Health Questionnaire 2 (PHQ-2); Generalized Anxiety Disorder 7 item scale (GAD-7); Pain Catastrophizing Scale (PCS); Insomnia Severity Index (ISI); Standard deviation (SD) p <. 05.
Pain
Pain was comprehensively measured using several self-report measures. First, the participants were asked questions about the frequency (how often and last time) as well as duration of chronic pain. Second, participants were given a list of body parts and asked to select all parts that were painful. Third, participants were asked to choose from a list of conditions that caused their pain. Composite scores were created for total number of body parts experiencing pain as well as number of painful conditions. Fourth, the participants filled out a modified version of the Brief Pain Inventory – Short Form (BPI) (28). Primary pain syndrome was derived from the initial BPI; if necessary, participants filled out a second BPI if they reported a secondary pain syndrome. Pain interference and pain relief questions from the BPI were answered with the participant taking into consideration all of their pain syndromes. Fifth, participants completed the Pain Catastrophizing Scale (PCS) - a 13-item scale that assesses important affective and cognitive aspects of pain (29). PCS total scores range from 0 to 52, with higher scores indicating heightened distress responses when exposed to aversive stimuli.
Mental Health
Participants completed brief validated screening questionnaires for depression (Patient Health Questionnaire (PHQ-2) (30) and anxiety (Generalized Anxiety Disorder 7-item scale (GAD-7) (31). PHQ-2 scores range from 0 to 6, with higher scores indicating a higher likelihood of having a depressive disorder. GAD-7 scores range from 0 to 21, with higher scores indicating a higher likelihood of having GAD. In addition, participants completed a validated measure of trait impulsivity (Barratt Impulsiveness Scale (BIS-11) (32). The BIS-11 contains thirty brief behavioral descriptions, and results are characterized in three subscales—attentional, motor, and non-planning. Lastly, participants completed the 5-item Insomnia Severity Index (ISI) (33–35); a validated measure of sleep problems. ISI total scores range from 0 to 28, with higher scores indicating a higher likelihood of clinical insomnia.
Opioid Use and Side Effects
Participants were asked two questions about past experience (yes/no) and duration of experience with opioid medications. In addition, participants reporting experience with opioids (N=174; 66% of sample) were also asked about frequency, level of distress and medication discontinuation as a result of common opioid side effects (36).
Statistical Analyses
Descriptive statistics were obtained by SOAPP-R category (<18 or ≥18) for each of the demographic, pain, mental health, insomnia, and opioid use variables. Where appropriate, Student’s t-tests were performed for continuous variables and chi-square or Fisher’s exact tests for categorical variables to examine differences by SOAPP-R category. In addition, chi-square or Fisher’s exact tests were performed to examine opioid side effects by SOAPP-R category. Area under the curve (AUC) values were calculated for the three series of BARQ questions to compare the overall likelihood of taking an analgesic medication given the expected duration of complete pain relief (3, 30, or 365 days) using methods previously described for probability discounting, with possible values ranging from 0 to 1 (37). Associations between the three AUC values and risk for opioid misuse were examined using two factor repeated measures analysis of variance (ANOVA) with SOAPP-R category, days of pain relief and a SOAPP-R × days of pain relief interaction term as factors. Tukey’s honestly significant difference (HSD) tests were used for planned post-hoc analyses.
As there were significant differences in AUC between respondents with high versus low SOAPP-R scores, regression analyses were performed to determine the independent effect of opioid misuse risk on likelihood to try novel analgesics. Generalized linear model (GLM) analyses were used with an identity link, Gaussian distribution, and robust standard error estimation to correct for non-normally distributed residuals of the regression model (STATA version 11.2; StataCorp, LLP, College Station, TX). Separate GLM analyses were done for the three AUC values. Predictors included SOAPP-R category, age, gender, sexual orientation, ethnicity, race, education, annual household income, duration of chronic pain, number of chronic pain diagnoses, last pain VAS, usual pain VAS, BPI severity rating for the primary pain, BPI interference VAS, whether or not a respondent had ever used opioids, PHQ-2 total score, GAD-7 total score, PCS total score, and ISI total score. A preliminary investigation into the association between predictors and each AUC value was conducted using univariate GLM analyses with the same link and distribution as the subsequent multivariate analyses. The univariate analyses were not used in deciding which predictors to include in multivariate models. To build the three final multivariate models, stepwise backward selection was used, where predictors were removed from the model if p>0.2. Each of the predictors used in the univariate analyses was placed in the multivariate model. The three final multivariate models were re-analyzed with all the included variables standardized in order to compare the relative effect of each variable on the outcome.
Results
Of the 300 completed surveys, 263 of them were retained for these analyses (Table 1). Those surveys dropped included five in which respondent did not report pain lasting > 3 months, eight persons who filled out the survey twice, and 24 persons who failed pre-specified quality control distractor questions. The following sections review the overall results and then separately by duration of pain relief specified for each series of questions (3, 30, or 365 days).
Overall Likelihood of Taking Novel Analgesic Medication
Figure 1a shows mean overall likelihood of taking a novel analgesic medication (as represented by AUC) by SOAPP-R category and by duration of expected pain relief. In repeated measures ANOVA of AUCs, SOAPP-R categorya and days of pain reliefb were statistically significant whereas day-by- SOAPP-R category trended toward significancec. In post-hoc analyses, participants with elevated SOAPP-R scores (≥18) showed significantly higher mean AUC, indicating a greater willingness to try the novel medication no matter the duration of pain relief provided compared to participants with low SOAPP-R scoresd. In participants with elevated SOAPP-R scores, AUC in the 365-day condition (.39) was significantly greater than the 3-day condition (.33) but not the 30-day condition (.36); whereas, there were no significant differences between AUC values in participants with SOAPP-R scores <18. These data suggest that CPP with greater risk for opioid misuse increased willingness to try a novel medication as the magnitude of expected pain relief increased. However, the effect size of the SOAPP-R score by days of pain relief interaction was small.
Figure 1. Pain Relief versus Addiction Risk in Probability of Taking a Novel Analgesic Medication.
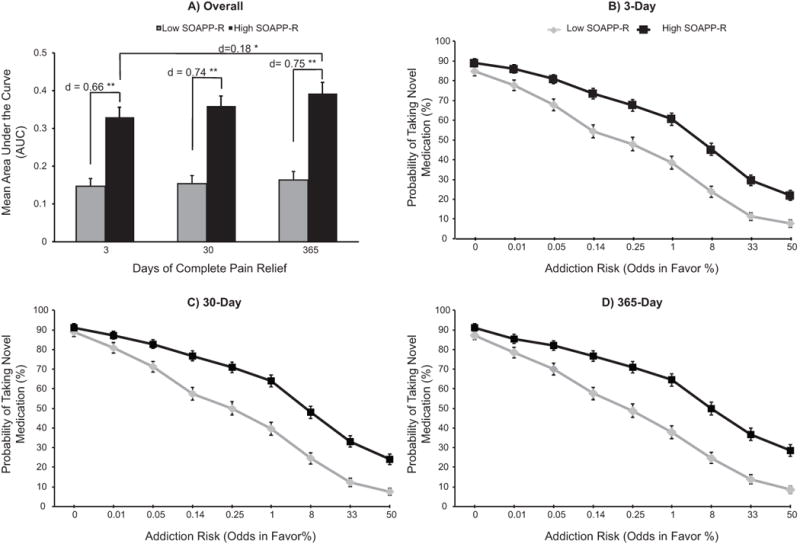
Section A) Mean AUC for Likelihood of Taking a Hypothetical Analgesic Medication by SOAPP-R Category. Participants answered questions for a novel analgesic mediation that provided B) three days of complete pain relief, C) 30 days of complete pain relief, or D) 365 days of complete pain relief. High versus low SOAPP-R scores are indicative of high versus low risk of opioid misuse, respectively. Data displayed by time of complete pain relief. Error bars represent standard error of the mean (SEM); *p<. 05; **p<. 001; d is Cohen’s d, a measure of the effect size. Abbreviations: area under the curve (AUC); Screener and Opioid Assessment for Patients in Pain-Revised (SOAPP-R). Greater AUC indicates greater self-reported likelihood of taking the medication.
3-Day Pain Relief
Figure 1b shows the effect of increasing addiction risk on the mean likelihood of taking a novel analgesic medication with 3 days of pain relief. Even with 0% chance of addiction risk, not all respondents were willing to try this novel analgesic. The steepest decline in likelihood occurred as the stated risk for addiction grew from 1 to 33% for both groups of respondents. In addition, there was an almost 3-fold difference in likelihood of taking a novel medication between SOAPP-R groups if 3 days of pain relief was associated with a 50:50 likelihood of developing addiction (21.9% vs. 7.6%).
In univariate GLM analysis of AUC values, several factors were associated with propensity to try a novel analgesic (Table 2); however, participants with higher levels of education were significantly less likely to try novel medication. In multivariate model building, gender, SOAPP-R scores, last pain VAS, ISI total score, number of pain diagnoses, BPI pain severity of primary pain condition, and the attentional and non-planning subscales of the BIS-11 were retained in final model (see Table 3 for results). Thus, respondents with greater risk for opioid misuse were more willing to try a novel analgesic medication that had addiction risk compared to persons with lower risk of opioid misuse, even after controlling for pain level and severity and personality measures of impulsivity.
Table 2.
Predicting BARQ AUC Scores: Univariate Analyses.
| 3-Day AUC | 30-day AUC | 365-day AUC | |||||
|---|---|---|---|---|---|---|---|
| Variable | β | 95% CI | β | 95% CI | β | 95% CI | |
| Age | −0.002 | −0.004, 0.001 | −0.002 | −0.004, 0.001 | −0.001 | −0.004, 0.002 | |
| Gender | Male (0) vs. Female (1) | −0.05 | −0.12, 0.03 | −0.06 | −0.14, 0.01 | −0.04 | −0.13, 0.05 |
| Race | Non-Caucasian (0) vs. Caucasian (1) | −0.001 | −0.10, 0.10 | −0.02 | −0.12, 0.08 | −0.003 | −0.10, 0.10 |
| Ethnicity | Non-Hispanic (0) vs. Hispanic (1) | 0.06 | −0.07, 0.18 | −0.01 | −0.12, 0.15 | −0.04 | −0.09, 0.17 |
| Sexual Orientation | Heterosexual (0) vs. Non-heterosexual (1) | 0.03 | −0.08, 0.15 | 0.07 | −0.06, 0.19 | 0.11 | −0.02, 0.25 |
| Past Opioid Use | 0.10 | 0.03, 0.16 | 0.09 | 0.02, 0.17 | 0.11 | 0.03, 0.19 | |
| SOAPP-R | Low (0) vs. High (1) | 0.18 | 0.12, 0.25 | 0.21 | 0.14, 0.27 | 0.23 | 0.16, 0.30 |
| Education | −0.05 | −0.09, −0.003 | −0.06 | −0.10, −0.01 | −0.07 | −0.13, −0.02 | |
| Income | −0.02 | −0.04, 0.005 | −0.02 | −0.04, 0.005 | −0.02 | −0.04, 0.002 | |
| Duration of Chronic Pain | 0.02 | −0.05, 0.10 | 0.04 | −0.02, 0.11 | 0.06 | −0.003, 0.13 | |
| Last Pain VAS | 0.03 | 0.01, 0.05 | 0.03 | 0.01, 0.05 | 0.02 | 0.003, 0.04 | |
| Usual Pain VAS | 0.03 | 0.01, 0.04 | 0.03 | 0.01, 0.05 | 0.03 | 0.01, 0.05 | |
| PHQ-2 | Total | 0.04 | 0.02, 0.06 | 0.04 | 0.02, 0.06 | 0.06 | 0.03, 0.08 |
| GAD-7 | Total | 0.01 | 0.005, 0.02 | 0.01 | 0.006, 0.02 | 0.01 | 0.008, 0.02 |
| PCS | Total | 0.005 | 0.002, 0.01 | 0.03 | 0.01, 0.04 | 0.03 | 0.02, 0.05 |
| ISI | Total | 0.01 | 0.008, 0.02 | 0.01 | 0.01, 0.02 | 0.01 | 0.01, 0.02 |
| Number Pain Dx | 0.02 | 0.006, 0.04 | 0.02 | 0.001, 0.04 | 0.03 | 0.004, 0.05 | |
| BPI | Interference | 0.04 | 0.02, 0.05 | 0.04 | 0.02, 0.05 | 0.04 | 0.03, 0.06 |
| BPI (Primary) | Worst | 0.03 | 0.02, 0.04 | 0.03 | 0.02, 0.05 | 0.03 | 0.02, 0.05 |
| Least | 0.02 | 0.005, 0.03 | 0.01 | 0.00, 0.03 | 0.01 | −0.003, 0.03 | |
| Average | 0.04 | 0.01, 0.06 | 0.03 | 0.01, 0.05 | 0.03 | 0.01, 0.06 | |
| Current | 0.03 | 0.01, 0.04 | 0.03 | 0.01, 0.04 | 0.03 | 0.01, 0.04 | |
| Severity | 0.04 | 0.02, 0.06 | 0.03 | 0.02, 0.05 | 0.03 | 0.02, 0.05 | |
| BIS-11 | Attentional | 0.01 | 0.001, 0.02 | 0.01 | 0.002, 0.02 | 0.01 | 0.003, 0.02 |
| Motor | 0.01 | 0.004, 0.02 | 0.01 | 0.006, 0.02 | 0.02 | 0.01, 0.03 | |
| Non-planning | 0.01 | 0.004, 0.02 | 0.01 | 0.006, 0.02 | 0.01 | 0.006, 0.02 | |
Univariate analysis for demographic and self-report measures used as predictors in multivariate GLM model building. Columns are divided by proposed time of complete pain relief. Abbreviations: BARQ Benefit vs. Addiction Risk Questionnaire (BARQ); Confidence Interval (CI); Screener and Opioid Assessment for Patients in Pain-Revised (SOAPP-R); Visual Analogue Scale (VAS); Brief Pain Index (BPI); Patient Health Questionnaire 2 (PHQ-2); Generalized Anxiety Disorder 7 item scale (GAD-7); Pain Catastrophizing Scale (PCS); Insomnia Severity Index (ISI); Diagnosis (Dx); Barratt Impulsiveness Scale version 11 (BIS-11); area under the curve (AUC). p <.05. p<0.10.
Table 3.
Predicting BARQ AUC Scores: Multivariate Analyses
| 3-day AUC | 30-day AUC | 365-day AUC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | β | 95% CI | Standardized Beta | β | 95% CI | Standardized Beta | β | 95% CI | Standardized Beta | |
| Gender | Male (0) vs. Female (1) | −0.05 | −0.12, 0.02 | −0.02 | −0.05 | −0.12, 0.02 | −0.02 | – | – | – |
| SOAPP-R | Low (0) vs. High (1) | 0.14 | 0.06, 0.21 | 0.07 | 0.15 | 0.07, 0.24 | 0.08 | 0.13 | 0.04, 0.22 | 0.07 |
| Education | – | – | – | – | – | – | −0.04 | −0.09, 0.01 | −0.03 | |
| Duration of chronic pain | – | – | – | – | – | – | 0.06 | 0.005, 0.12 | 0.03 | |
| Last Pain VAS | – | – | – | 0.02 | 0.003, 0.04 | 0.04 | – | – | – | |
| PHQ-2 | Total | – | – | – | – | – | – | 0.03 | 0.00, 0.05 | 0.05 |
| ISI | Total | 0.01 | −0.001, 0.12 | 0.04 | 0.01 | 0.00, 0.01 | 0.04 | – | – | – |
| Number Pain Dx | 0.02 | 0.00, 0.04 | 0.03 | 0.01 | −0.004, 0.03 | 0.03 | – | – | – | |
| BPI (Primary Pain) | Severity | 0.02 | 0.004, 0.04 | 0.04 | – | – | – | – | – | – |
| Interference | – | – | – | – | – | – | 0.02 | 0.01, 0.04 | 0.05 | |
| BIS-11 | Attentional | −0.01 | −0.02, 0.003 | −0.03 | −0.01 | −0.02, 0.002 | −0.04 | −0.01 | −0.03, −0.001 | −0.07 |
| Motor | – | – | – | – | – | – | 0.01 | 0.00, 0.02 | 0.05 | |
| Non-planning | 0.01 | 0.00, 0.01 | 0.04 | 0.01 | 0.003, 0.02 | 0.06 | 0.01 | 0.00, 0.01 | 0.05 | |
Multivariate analysis for final model between participants at high versus low risk for opioid misuse. Abbreviations: BARQ Benefit vs. Addiction Risk Questionnaire (BARQ); Confidence Interval (CI); area under the curve (AUC); Screener and Opioid Assessment for Patients in Pain-Revised (SOAPP-R); Brief Pain Inventory (BPI); Insomnia Severity Index (ISI); Diagnosis (Dx); Barratt Impulsiveness Scale version 11 (BIS-11). p <.05. p<0.10. If “–“ appears in the column, that indicates the variable was not included in the final model.
30-Day Pain Relief
Figure 1c shows the effect of increasing addiction risk on mean likelihood of taking a novel once daily oral analgesic medication with 30 days of complete pain relief. These curves are similar to the 3-day pain relief curves, with high SOAPP-R respondents more likely to take the novel medication at each addiction risk >0%.
In univariate GLM analysis of AUC values (Table 2), the same variables that were significant for 3-day were significant for novel medication providing 30-days of complete pain relief. In multivariate model building, gender, SOAPP-R scores, last pain VAS, ISI total score, and number of pain diagnoses were retained in final model (see Table 3 for results).
365-Day Pain Relief
Figure 1d shows the effect of increasing addiction risk on mean likelihood of taking a novel analgesic medication with 365 days (1 year) of complete pain relief. As in the 3-day and 30-day conditions, respondents with high SOAPP-R scores were more likely to take the novel medication at each addiction risk >0%.
In univariate GLM analysis of AUC values, most variables that were significant in 3-day and 30-day analyses remained significant (Table 2). In multivariate model building, gender, SOAPP-R score, education, duration of chronic pain, PHQ-2 total score, and BPI pain interference composite score were retained in the final model (see Table 3 for results).
Prior Opioid Side Effects
Of the 174 respondents who had a past history using opioid medications, the most common side effects reported were drowsiness, lightheaded/dizzy, and fatigued/weak (Table 4). Persons with high SOAPP-R scores reported overall more total side effects (high vs. low mean (95% CI): 7.6 (7.0, 8.3) vs. 6 (5.3, 6.6), p=0.001), but they reported they were less likely to stop the opioids due to side effects compared to persons with low scores. There were statistically significant differences between groups in levels of distress from side effects (Table 4). Only nausea was associated with a significantly lower percentage of high vs. low SOAPP-R respondents in stopping opioid use (Table 4). In exploratory GLM regression analysis of AUC values, final models were re-run on only participants with past opioid exposure with the addition of number of opioid side effects. The number of past opioid side effects was associated with significantly reduced willingness to try novel pain medication with 30 days and 365 days of complete pain relief but not 3-dayse.
Table 4.
Opioid Side Effects Questionnaire by SOAPP-R Status.
| A. Low SOAPP-R (N=72) | |||||||
|---|---|---|---|---|---|---|---|
| How much did the side effect distress or bother you while on opioids? | |||||||
| Did Not Have Side Effect (%) | Rarely (%) | Occasionally (%) | Frequently (%) | Almost Continually (%) | Missing (%) | Stop Using Due to Side Effect? (% Yes) | |
|
|
|||||||
| Nausea | 47 | 8 | 17 | 18 | 6 | 4 | 24 |
| Vomiting | 65 | 6 | 7 | 7 | 3 | 13 | 14 |
| Constipation | 42 | 24 | 15 | 11 | 4 | 4 | 10 |
| Difficulty Passing Urine | 79 | 4 | 4 | 0 | 0 | 13 | 4 |
| Difficulty Concentrating | 49 | 18 | 6 | 8 | 11 | 8 | 18 |
| Drowsiness | 29 | 22 | 22 | 15 | 10 | 1 | 21 |
| Feeling Lightheaded/Dizzy | 44 | 13 | 13 | 14 | 11 | 6 | 29 |
| Feeling Confused | 63 | 6 | 10 | 6 | 8 | 8 | 19 |
| Feeling Fatigued/Weak | 44 | 22 | 14 | 4 | 8 | 7 | 14 |
| Itchiness | 64 | 3 | 10 | 7 | 4 | 13 | 11 |
| Dry Mouth | 53 | 14 | 13 | 6 | 1 | 14 | 6 |
| Headache | 61 | 7 | 10 | 6 | 3 | 14 | 17 |
| Feeling Drunk/High | 50 | 14 | 6 | 4 | 15 | 11 | 28 |
| B. High SOAPP-R (N=102) | |||||||
|---|---|---|---|---|---|---|---|
| How much did the side effect distress or bother you while on opioids? | |||||||
| Did Not Have Side Effect (%) | Rarely (%) | Occasionally (%) | Frequently (%) | Almost Continually (%) | Missing (%) | Stop Using Due to Side Effect? (% Yes) | |
|
|
|||||||
| Nausea | 30 | 19 | 20 | 15 | 10 | 7 | 11 |
| Vomiting | 53 | 12 | 5 | 8 | 11 | 12 | 9 |
| Constipation | 31 | 18 | 16 | 17 | 7 | 12 | 9 |
| Difficulty Passing Urine | 69 | 7 | 6 | 5 | 2 | 12 | 5 |
| Difficulty Concentrating | 27 | 20 | 21 | 14 | 9 | 10 | 16 |
| Drowsiness | 22 | 16 | 28 | 15 | 11 | 9 | 19 |
| Feeling Lightheaded/Dizzy | 27 | 18 | 25 | 14 | 9 | 8 | 20 |
| Feeling Confused | 37 | 18 | 15 | 9 | 10 | 12 | 15 |
| Feeling Fatigued/Weak | 27 | 15 | 18 | 21 | 11 | 9 | 15 |
| Itchiness | 52 | 15 | 12 | 7 | 5 | 10 | 11 |
| Dry Mouth | 49 | 21 | 10 | 5 | 6 | 10 | 10 |
| Headache | 52 | 11 | 10 | 11 | 4 | 13 | 11 |
| Feeling Drunk/High | 38 | 16 | 17 | 13 | 10 | 7 | 21 |
|
|
|||||||
Not all participants had previously used prescription opioids to manage pain (n=174 out of the total sample n=263). Participants are split into low risk for opioid misuse (Low SOAPP-R) and high risk for opioid misuse (High SOAPP-R). p <.05 comparing Low vs. High SOAPP-R (Screener and Opioid Assessment for Patients with Pain-Revised).
Discussion
The central finding of these analyses is that self-identified CPP that are at high risk for opioid misuse (SOAPP-R score ≥18) are more likely to try novel analgesic medications at a range of addiction risk probabilities when compared to individuals at low risk for opioid misuse. In multivariate analysis, the association between probability discounting AUC and SOAPP-R scores remained significant even when accounting for respondent’s pain severity, pain interference, and pain catastrophizing (Tables 3–5). High scores on the non-planning BIS-11 subscale were also associated with propensity to try novel analgesics (Table 1), suggesting that patients with higher trait impulsivity are more likely to discount the probability of becoming addicted as a potential medication side effect. In addition, self-identified CPP that had previously experienced other opioid side effects were less likely to try a novel analgesic, even though the novel analgesic medications proposed in this study was not identified as an opioid.
Individuals at high risk for opioid misuse might be more prone to risky decision-making in general (17, 23), as a prior paper derived from these same survey respondents found greater discounting of delayed punishments (both pain and money related) when comparing CPP with high versus low risk for opioid misuse (21). From a clinical standpoint, high risk individuals might be more likely to ask about or try to influence physicians in seeking medications that have high abuse potential if they view these medications as a reward (38). Indeed, high-risk individuals have more risk tolerance for every hypothetical proposition in the BARQ (Figure 1). Trait factors of impulsivity, measured by the BIS-11, were elevated in CPP at high risk for opioid misuse (Table 1). In addition, scores on the BIS-11 were also associated with increased willingness to try a novel analgesic (Table 2). Although a small effect, CPP at high risk for opioid misuse were more likely to try a novel analgesic with elevated addiction risk if the duration of pain relief was increased (thus, increased reward); this phenomenon was not found in low risk CPP suggesting that low risk CPP may make decisions based mostly on risk of addiction (Figure 1d). It is well established that trait impulsivity is predictive of development of substance use disorders (SUDs) (39, 40), a finding that has recently been extended to the development of opioid misuse in CPP (41). Similarly, research on probability discounting has echoed the relationship between SUDs and risky decision-making (22, 42). For example, a recent study by Johnson et al. found that alcohol use increases the probability discounting of acquiring a sexually transmitted disease by forgoing condom-protected sex (42). The probability discounting approach used in our study is the first to examine choice of medication in self-identified CPP, and might be comparable to discrete choice experiments insofar as the goal is to establish trade-offs that the participant is willing to make, i.e. weighing risk against reward (43). The current study paradigm weighs the probability of risk of addiction in a way that is not time dependent and appears to have more influence over the decision-making process than the reward of protracted pain relief (see Figure 1d).
In addition to state/trait measurements, previous experience with opioid side effects was associated with willingness to try a novel analgesic that provided 30 or 365 days of complete pain relief. More specifically, CPP at low risk for opioid misuse reported fewer side effects during prior opioid use; however, these same individuals were more likely to have stopped taking opioids in the past due to a side effect (e.g. nausea; Table 4). These results point to the importance that prior experience have on trying a new analgesic medication.
Previous clinical research has shown that CPP have a high desire for information regarding their care, particularly in premedication visits (44). However, physicians often fail to collect and relay critical information regarding patient needs and experiences. Many physicians may be unsure how to address risk factors when interacting with CPP. For instance, primary care physicians often feel ill prepared to prescribe opioids and may avoid the topic all together during patient visits (45). Similarly, physicians in community clinical settings frequently cite issues such as lack of self-management and abuse potential as reasons why they are wary of prescribing opioids long-term to CPP (46). While physicians should seek to inform patients about all risks, it is likely that increased emphasis of the abuse potential in the informed consent process may actually decrease the number of low risk CPP willing to utilize opioids to manage chronic pain, even though they would be at lower risk for abusing opioids. Engaging in open dialogue during consent could be used as a way to guide low risk individuals into effective treatments, while high risk individuals can be flagged for monitoring from the beginning of treatment or offered alternative treatments than opioids. Individuals that are eager to try a medication despite elevated addiction risk might be another indication that they are at high risk for medication misuse.
This study has some limitations. The use of AMT workers may or may not be representative of the chronic pain population as a whole, although the participant demographics and variety of chronic pain syndromes are similar to other large samples (47, 48). In addition, surveys were done anonymously, without verification from a clinician or treatment center of a chronic pain diagnosis; on the other hand, this procedure did allow sampling several types of self-identified CPP that might not be treated in specialty clinics, and anonymity is likely to facilitate truthful responding. It is also possible that the results from this study are at least partially influenced by common-method variance, in that both the outcome variable and predictor of interest (SOAPP-R) were self-reported during an anonymous survey. Also, our survey did not define addiction, although most primary care physicians also do not define addiction in their practice, which suggests that leaving the definition of addiction to the participant replicates real-world decision-making. Lastly, we did not define the novel medication as an opioid; therefore, differing results may have occurred if the class of novel medication had been defined.
This study is a promising step in understanding the decision-making process that underlies medication choice in a chronic pain population. Individuals at the highest risk for opioid misuse are also the most likely to discount addiction risk when choosing a pharmacotherapy. Other factors, such as trait impulsivity, pain interference and previous experience with opioid medications, also shape this decision-making process. Physicians should seek to tailor informed consent discussions in a way that cautions the use of medications with heightened abuse liability; our data show that individuals at high risk for opioid misuse might gravitate towards medications with high abuse potential, while relatively low risk individuals will be less likely to utilize them. However it is the low risk individuals that could possibly benefit from these medications without misusing them. In addition, the study provides evidence that the FDA labeling process for immediate release opioids (49) (which details both risks and benefits) may have unintended consequences as the emphasis of addiction in the black box warning may increase the proportion of opioid patients at high risk for opioid misuse receiving prescriptions for these medications. Future controlled investigations on drug labeling are needed.
Supplementary Material
Acknowledgments
We would like to acknowledge Sarah Passio for help in survey development and data management as well as the AMT workers who completed the surveys.
The work described in this manuscript was funded by the National Institute on Drug Abuse (DA029609, DA07209). DAT has received medication supplies from Indivior (formerly Reckitt Benckiser Pharmaceuticals) for an investigator initiated study, is site PI for a clinical trial sponsored by Alkermes, and provided consulting services for AstraZeneca and Theravance. In the past two years, ECS has providing consulting services to the following pharmaceutical companies: DemeRx, Jazz, Reckitt Benckiser, Relmada, Transcept, and Zogenix. In addition, ECS has served on an advisory panel for the Oak Group.
Footnotes
Declarations of Interest: There are no conflicts to report for ASH, PSJ, MTS, RRE, and MWJ.
F(1,261) = 37.22; p<0.0001; partial η2 = 0.58, 95% CI = 0.53, 0.62
F(2,522) = 8.91; p<0.001; partial η2 = 0.03, 95% CI 0.01, 0.07
F(2,522) = 3.00; p=0.051; partial η2=0.01, 95% CI 0, 0.03
(3-day: Cohen’s d = 0.66, 95% CI 0.63, 0.69; 30-day: d = 0.74, 95% CI 0.71, 0.78; 365-day: d = 0.75, 95% CI 0.72, 0.79)
(3-days: β = −0.01, 95% CI −0.03, 0.00, p=0.07; 30 days: β = −0.02, 95% CI −0.03, −0.01, p=0.02; 365 days: β = −0.02, 95% CI −0.03, −0.003, p=0.02)
References
- 1.National Center for Health Statistics, Centers for Disease Control and Prevention. Health, United States, 2013, with special feature on prescription drugs. Government Printing Office; Hyattsville, MD: 2014. [cited 2017, May10]. Available from: https://www.cdc.gov/nchs/data/hus/hus13.pdf. [PubMed] [Google Scholar]
- 2.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–63. doi: 10.1056/NEJMra1508490. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–92. [PubMed] [Google Scholar]
- 4.Rudd RA, Aleshire N, Zibbell JE, Matthew Gladden R. Increases in drug and opioid overdose deaths—United States, 2000–2014. Am J Transplant. 2016;16:1323–7. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 5.Adler E. Heroin, prostitution: If this mom and daughter can beat addiction, can America, too? Kansas City Star. 2017 [cited 2017, May 11]. Available from: http://www.kansascity.com/news/local/article143441464.html-storylink=cpy.
- 6.Calabresi M. They’re the most powerful painkillers ever invented. And they’re creating the worst addiction crisis America has ever seen. Time. 2015 Cover Story. [Google Scholar]
- 7.Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131,146. e5. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, et al. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage. 2004;28:250–8. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Mühlbacher AC, Juhnke C. Patient preferences versus physicians’ judgement: does it make a difference in healthcare decision making? Appl Health Econ Health Policy. 2013;11:163–80. doi: 10.1007/s40258-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 10.Anastassopoulos KP, Tapia CI, Baik R, Moskowitz B, Kim MS. Reported side effects, bother, satisfaction, and adherence in patients taking hydrocodone for non-cancer pain. J Opioid Manag. 2013;9:97–109. doi: 10.5055/jom.2012.0151. [DOI] [PubMed] [Google Scholar]
- 11.McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. 2003;4:231–56. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 12.Allen MJ, Asbridge MM, MacDougall PC, Furlan AD, Tugalev O. Self-reported practices in opioid management of chronic noncancer pain: a survey of Canadian family physicians. Pain Res Manag. 2013;18:177–84. doi: 10.1155/2013/528645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nwokeji ED, Rascati KL, Brown CM, Eisenberg A. Influences of attitudes on family physicians’ willingness to prescribe long-acting opioid analgesics for patients with chronic nonmalignant pain. Clin Ther. 2007;29:2589–602. doi: 10.1016/j.clinthera.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Mühlbacher AC, Junker U, Juhnke C, Stemmler E, Kohlmann T, Leverkus F, et al. Chronic pain patients’ treatment preferences: a discrete-choice experiment. Eur J Health Econ. 2015;16:613–28. doi: 10.1007/s10198-014-0614-4. [DOI] [PubMed] [Google Scholar]
- 15.Mühlbacher A, Johnson FR. Choice experiments to quantify preferences for health and healthcare: state of the practice. Appl Health Econ Health Policy. 2016;14:253–66. doi: 10.1007/s40258-016-0232-7. [DOI] [PubMed] [Google Scholar]
- 16.Du W, Green L, Myerson J. Cross-cultural comparisons of discounting delayed and probabilistic rewards. Psychol Rec. 2002;52:479. [Google Scholar]
- 17.Estle SJ, Green L, Myerson J, Holt DD. Discounting of monetary and directly consumable rewards. Psychol Sci. 2007;18:58–63. doi: 10.1111/j.1467-9280.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 18.Arch JJ, Carr AL. Using Mechanical Turk for research on cancer survivors. Psycho-Oncology. 2016 doi: 10.1002/pon.4173. [DOI] [PubMed] [Google Scholar]
- 19.Strickland JC, Stoops WW. Perceptions of research risk and undue influence: Implications for ethics of research conducted with cocaine users. Drug Alcohol Depend. 2015;156:304–10. doi: 10.1016/j.drugalcdep.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Tejwani R, Wang HS, Lloyd JC, Kokorowski PJ, Nelson CP, Routh JC. Utility estimation for pediatric vesicoureteral reflux: methodological considerations using an online survey platform. J Urol. 2017;197:805–10. doi: 10.1016/j.juro.2016.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tompkins DA, Johnson PS, Smith MT, Strain EC, Edwards RR, Johnson MW. Temporal preference in individuals reporting chronic pain: discounting of delayed pain-related and monetary outcomes. Pain. 2016;157:1724–32. doi: 10.1097/j.pain.0000000000000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson MW, Johnson PS, Herrmann ES, Sweeney MM. Delay and probability discounting of sexual and monetary outcomes in individuals with cocaine use disorders and matched controls. PloS one. 2015;10:e0128641. doi: 10.1371/journal.pone.0128641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What Percentage of Chronic Nonmalignant Pain Patients Exposed to Chronic Opioid Analgesic Therapy Develop Abuse/Addiction and/or Aberrant Drug-Related Behaviors? A Structured Evidence-Based Review. Pain Med. 2008;9:444–59. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 24.Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, et al. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain. 2008;9:360–72. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-Validation of a Screener to Predict Opioid Misuse in Chronic Pain Patients (SOAPP-R) J Addict Med. 2009;3:66–73. doi: 10.1097/ADM.0b013e31818e41da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain. 2010;150:390–400. doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendoza T, Mayne T, Rublee D, Cleeland C. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10:353–61. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524. [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 32.Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–36. [PubMed] [Google Scholar]
- 34.Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population: influence of previous complaints of insomnia. Arch Intern Med. 1992;152:1634–7. [PubMed] [Google Scholar]
- 35.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao SZ, Chung F, Hanna DB, Raymundo AL, Cheung RY, Chen C. Dose-response relationship between opioid use and adverse effects after ambulatory surgery. J Pain Symptom Manage. 2004;28:35–46. doi: 10.1016/j.jpainsymman.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–43. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–55. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- 41.Vest N, Reynolds CJ, Tragesser SL. Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addict Behav. 2016;60:184–90. doi: 10.1016/j.addbeh.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Johnson PS, Sweeney MM, Herrmann ES, Johnson MW. Alcohol Increases Delay and Probability Discounting of Condom-Protected Sex: A Novel Vector for Alcohol-Related HIV Transmission. Alcohol Clin Exp Res. 2016;40:1339–50. doi: 10.1111/acer.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali S, Ronaldson S. Ordinal preference elicitation methods in health economics and health services research: using discrete choice experiments and ranking methods. Br Med Bull. 2012;103:21–44. doi: 10.1093/bmb/lds020. [DOI] [PubMed] [Google Scholar]
- 44.Spies C, Schulz C, Weiß-Gerlach E, Neuner B, Neumann T, Von Dossow V, et al. Preferences for shared decision making in chronic pain patients compared with patients during a premedication visit. Acta Anaesthesiol Scand. 2006;50:1019–26. doi: 10.1111/j.1399-6576.2006.01097.x. [DOI] [PubMed] [Google Scholar]
- 45.Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8:573–84. doi: 10.1111/j.1526-4637.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 46.Upshur CC, Luckmann RS, Savageau JA. Primary care provider concerns about management of chronic pain in community clinic populations. J Gen Intern Med. 2006;21:652–5. doi: 10.1111/j.1525-1497.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9:803–12. doi: 10.1111/j.1526-4637.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 48.Melnikova I. Pain market. Nat Rev Drug Discov. 2010;9:589–90. doi: 10.1038/nrd3226. [DOI] [PubMed] [Google Scholar]
- 49.Food and Drug Administration. FDA Boxed Warning for Immediate-Release Opioids. J Pain Palliat Care Pharmacother. 2016;30:141–5. doi: 10.3109/15360288.2016.1173762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


