Abstract
Multiple sclerosis (MS) is a classical inflammatory demyelinating disease of the central nervous system (CNS). Microglia are the main resident immune cells in the CNS and are closely associated with the pathogenesis of MS. In the present study, we found that miR-30a was highly expressed in jellyfish-like microglia in chronic active lesions of MS patients, as well as in the microglia of mice with experimental autoimmune encephalomyelitis (EAE) at the chronic phase. In vitro, the conditioned supernatant of mouse microglia overexpressing miR-30a promoted the apoptosis of oligodendrocyte precursor cells (OPCs), and inhibited OPC differentiation. In vivo, overexpressing miR-30a in transplanted microglia exacerbated the progression of EAE. Overexpression and knock-down experiments in primary cultured mouse microglia showed that miR-30a increased the expression of IL-1β and iNOS, which are pro-inflammatory, while inhibiting the expression of Ym-1 and CD206. Mechanistically, miR-30a inhibited the expression of Ppargc1b, which is the co-activator of peroxisome proliferator-activated receptor gamma, resulting in pro-inflammatory effects. Our work shows that miR-30a is an important regulator of the inflammatory response in microglia, and may be a promising therapeutic target for inflammatory diseases like MS in the CNS.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0153-y) contains supplementary material, which is available to authorized users.
Keywords: Multiple sclerosis, Experimental autoimmune encephalomyelitis, MiR-30a, Microglia, Inflammation
Introduction
Multiple sclerosis (MS) is a multifocal, inflammatory demyelinating disease which mainly affects the white matter of the central nervous system (CNS). It occurs mainly in young and middle-aged adults and can cause functional impairments of multiple systems [1]. In the early stages, MS often appears in a relapsing-remitting pattern, but in the later stage the disease develops into a secondary progressive phase without remission [2]. Currently, no effective treatment is available for progressive MS [3]. In the development of MS, the immune response plays a key role in the processes of demyelination and nerve injury, which jointly promote disease progression [4, 5]. One important player in the immune response of MS is microglia [6–8]. Microglia are both the main innate immune cells and the activators of acquired immunity in the CNS. Studies have shown that microglia are activated in the early stage of MS while no significant T cell infiltration occurs, suggesting that microglia are involved in the early occurrence of MS [9, 10]. As a possible initiator, an important inflammatory participant, and a vital post-injury agent of repair, microglia have attracted increasing attention in MS.
The complex roles of microglia in MS derive from their various functions, among which the multidirectional inflammatory response is a major feature [11]. Microglia can secrete IL-1β, NO, and other factors that promote inflammation and aggravate tissue damage, but can also express arginase-1, CD206, and other factors and receptors to attenuate inflammation and enhance tissue repair and the removal of debris [6, 12, 13]. The role of microglia in a specific phase of the inflammatory process is regulated by a complex molecular network [14, 15]. The abnormal expression of some regulatory molecules may contribute to MS.
MicroRNAs are a class of non-coding RNAs ~22 bases long [16]. Some microRNAs participate in the regulation of microglial functions, like miR-124 and miR-155 [17, 18]. MiR-30a is a member of the miR-30 family (a–e), which is highly conserved between zebrafish and human, and plays significant roles in zebrafish visceral development, human tumorigenesis, and T cell differentiation [19–22]. However, the expression pattern and functional role of miR-30a in microglia are unknown.
In this work, we set out to investigate the expression of miR-30a in microglia/macrophages from MS patients and in mice with experimental autoimmune encephalomyelitis (EAE) and its possible roles and mechanisms of action, expecting to find a new therapeutic target for MS.
Materials and Methods
Cell Cultures
Neonatal C57BL/6 mice were sacrificed by carbon dioxide anesthesia to obtain microglia. The cerebra were removed, homogenized, and digested before cultivation in T75 cell culture flasks. After 10–14 days of growth in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS, Gibco, Grand Island, NY), cultures were shaken for 4–6 h at 180 rpm at 37 °C and microglia in the supernatant were collected. Primary cultured microglia were stimulated with IFN-γ (100 ng/mL, PeproTech, Rocky Hill, NJ), IL-4 (10 ng/mL, PeproTech), IL-17 (10 ng/mL, PeproTech), or TGF-β (5 ng/mL, PeproTech) for 24 h, and the expression of miR-30a was assessed by quantitative PCR.
Neonatal Sprague–Dawley rats were sacrificed by carbon dioxide anesthesia to obtain oligodendrocyte precursor cells (OPCs). The cortices were removed, homogenized, and digested to provide mixed cortical glial cell cultures. After 8–10 days of growth in DMEM containing 10% FBS, cultures were shaken for 1 h at 180 rpm at 37 °C to remove microglia. Then the cultures were shaken for another 14 h at 200 rpm at 37 °C after replacing the medium to collect OPCs.
OPCs were co-cultured with microglia-conditioned media at a 1:1 ratio.
Animal Experiments
The animal experiments in this study were carried out in adherence with the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals and were approved by the Animal Experimentation Ethics Committee of the Second Military Medical University. Female C57BL/6 mice (at 6 weeks) were purchased from the Shanghai Laboratory Animal Center (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai) and maintained for 2–4 weeks before EAE induction. MOG35-55 (myelin oligodendrocyte glycoprotein, 150 ng per mouse, GL Biochem, Shanghai, China) in incomplete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO) containing heat-killed Mycobacterium tuberculosis (5 mg/mL, H37Ra strain; Difco, Detroit, MI) was subcutaneously injected at 3–4 points on the backs of mice to be immunized, and that day was defined as day 0. On days 0 and 2, pertussis toxin (250 ng in 200 μL PBS per mouse, Calbiochem-EMD Chemicals, San Diego, CA) was administered intraperitoneally. All procedures involving mice were planned and performed in such a way as to minimize suffering. Clinical manifestations were blindly examined daily. The clinical score was determined on a scale of 0–5 as follows: 0, no clinical signs; 1, paralyzed tail; 2, ataxia and/or paresis of hindlimbs; 3, paraplegia; 4, paraplegia with forelimb weakness or paralysis; and 5, moribund state or death.
For transplantation of microglia into the EAE model, the mouse was fixed in a stereotaxic apparatus and a small hole was drilled into the skull over the right lateral ventricle. Cells (1 × 106 in 10 μL) were infused with a microinjector at 1 μL/min.
Lentivirus Transduction and Electroporation
For lentivirus transduction, the sequence of primary miR-30a was synthesized and ligated into the GV271 plasmid (GeneChem, Shanghai, China). The cloned sequence starts from “GTTTACAGAATGTTGC” and ends at “TCTTTGATTTATTTTT”. The titer of viral particles was 0.5 × 109–1 × 109 transducing units/mL. Lentiviral particles were added to cultured microglia at a multiplicity of infection (MOI) of 10 and the supernatant was changed 12 h after infection.
For electroporation, agomiR-30a and antagomiR-30a were synthesized by GenePharma (Shanghai, China). Mouse microglia were collected and transfected using nucleofection (programme Y-001, Amaxa, Basel, Switzerland) with agomiR-30a, or antagomiR-30a, or the “scrambled” control.
Immunohistofluorescence Staining (IHF)
Generally, cells and tissue sections were fixed, permeabilized, blocked, and incubated with primary antibodies (MBP, MAB382, Chemicon, Temecula, CA; NG2, AB5320, Chemicon, Temecula, CA; GFAP, G3839, Sigma-Aldrich, St. Louis, MO; Iba1, 019-19741, Wako, Osaka, Japan; Olig1, MAB5540, Millipore, Billerica, MA; PCNA, BM0104, Boster Biological Technology, Wuhan, China) overnight at 4 °C, followed by 2 h incubation with TRITC- or FITC-conjugated secondary antibody (Jackson Immuno Research, West Grove, PA) and counterstained with Hoechst (Sigma-Aldrich, St. Louis, MO). In EAE mice, after terminal anesthesia with 10% chloral hydrate and perfusion through the left ventricle with PBS and 4% paraformaldehyde (PFA), spinal cords were isolated and post-fixed in 4% PFA. The spinal cords from EAE mice and brain tissues of MS patients obtained from the Netherlands Brain Bank (NBB) were embedded in paraffin and cut into 5 μm thick sections. Fluorescence images were captured using a fluorescence microscope (DXM, Nikon, Tokyo, Japan) or a confocal microscope (Leica, Buffalo Grove, IL).
For statistical analysis, the numbers of positive cells and total cells in one section were counted and the ratio of positive/total cells was calculated. At least 10 sections were counted for each specimen and 3 specimens were used in one group. All counting work was done blindly by two independent researchers.
Fluorescence In Situ Hybridization (FISH)
The LNATM miR-30a probe used for FISH was from Exiqon (38468-05, Vedbaek, Denmark). The TSATM-Plus Fluorescein System was from PerkinElmer (NEL741, Alpharetta, GA). FISH was carried out according to the manufacturer’s instructions, after which IHF was carried out as above. For detailed procedures please refer to a previous report [23].
Viability Assay
The viability of OPCs was evaluated by measuring lactate dehydrogenase (LDH) activity in the medium using a CytoTox nonradioactive assay (Promega, Madison, WI) according to the manufacturer’s protocol.
TUNEL Assays
TUNEL assays were carried out using In Situ Cell Death Detection Kit TMR red (12156, Roche, Indianapolis, IN), according to the manufacturer’s instructions. Briefly, the cells or specimens were fixed and permeabilized. Then the TUNEL reaction mixture was added and incubated in a humidified 37 °C chamber for 1 h. The nuclei were labeled with Hoechst 33342 (Sigma-Aldrich). The percentage of TUNEL-labeled cells versus Hoechst-labeled cells was calculated.
Flow Cytometry
To isolate microglia from EAE mice, the mice were sacrificed and the hindbrain and spinal cord were removed, homogenized, and digested. After filtration using a 100 μm aperture strainer, cells were separated by gradient centrifugation using Percoll (GE Healthcare, Marlborough, MA) and the majority of mononuclear cells were collected. After incubation with CD16/CD32 antibody (BD Biosciences, San Diego, CA) for blockade, mononuclear cells were stained with PE-CD45 (BD Biosciences) and FITC-CD11b antibodies (BD Biosciences). CD11b+CD45L cells were sorted as microglia on a Moflo XDP (Beckman Coulter, Indianapolis, IN) and assessed.
RNA Isolation and Quantitative PCR
Cells were lysed with TRIzol reagent (Invitrogen, Carlsbad, CA) and total RNA was extracted according to the manufacturer’s instructions. Then first-strand cDNA was synthesized using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific Fermentas, Logan, UT) and quantitative PCR (qPCR) was performed using the SYBR Green Real-time PCR Master Mix (Toyobo, Osaka, Japan) or the TaqMan Gene Expression Assay (sno202 and miR-30a, Life Technologies, Carlsbad, CA) according to the manufacturer’s instructions. Gene expression was normalized to a standard housekeeping gene (GAPDH or sno202) using the ΔΔCT method. The primer pairs were as follows:
- Ppargc1b
-
5′-AGAGAGAAAGACTTGAATCTGC-3′ (sense);
5′-ACAGCATGGAAACTCACGGAC-3′ (anti-sense);
- IL-1β
-
5′-TTCAGGCAGGCAGTATCA-3′ (sense);
5′-GTCACACACCAGCAGGTTAT-3′ (anti-sense);
- iNOS
-
5′-TTGACGCTCGGAACTGTAG-3′ (sense);
5′-GACCTGATGTTGCCATTGT-3′ (anti-sense);
- CD206
-
5′-GCTTCCGTCACCCTGTATG-3′ (sense);
5′-CTCCACAATCCCGAACCT-3′ (anti-sense);
- Ym-1
-
5′-TACTCCTCAGAACCGTCAGAT-3′ (sense);
5′-CATTTCCTTCACCAGAACAC-3′ (anti-sense);
- GAPDH
-
5′-TCAACGACCCCTTCATTGACC-3′ (sense);
5′-CTTCCCGTTGATGACAAGCTTC-3′ (anti-sense).
Western Blot and ELISA
For western blot, primary cultured microglia transfected with lentivirus were homogenized in NP40 buffer (Beyotime, Shanghai, China) supplemented with a protease inhibitor cocktail (Roche). The cell lysates were subjected to western blot using the anti-Ppargc1b (1:1000, Abcam, Cambridge, UK) and HRP-conjugated anti-GAPDH (Kangcheng, Shanghai, China) antibodies. The protein bands were analyzed using Image Lab (Bio-Rad, Hercules, CA).
The TNF-α and IL-1β levels in the supernatants were assessed using ELISA kits according to the manufacturer’s instructions (EK2822 for TNF-α, EK201B2 for IL-1β, Multi Sciences, Hangzhou, China). The sample concentrations were calculated using an equation generated from the standard curve.
Luciferase
We used a dual luciferase reporter system as follows. The 3′ UTR of Ppargc1b was cloned after luciferase in the pGL3-Basic vector and the binding between miR-30a and the 3′ UTR of Ppargc1b linked to the luciferase mRNA impeded the translation of luciferase. The binding between miR-30a and the 3′ UTR of IRF4 was shown by a decrease in the fluorescence intensity. HEK293T cells were maintained in DMEM containing 10% FBS (Gibco, Grand Island, NY), and the luciferase reporter gene construct (500 ng per well), the pRL-SV40 Renilla luciferase construct (1 ng per well; for normalization) and Agomir-30a (500 ng per well, GenePharma, Shanghai, China) were all co-transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) when the cells reached 30%–40% confluence. Cell extracts were prepared 24 h after transfection and the luciferase activity was measured with a Dual-Luciferase Reporter Assay system (Promega, Madison, WI).
Hematoxylin and Eosin (H&E) and Luxol Fast Blue (LFB) Staining
The lumbar spinal cords of EAE mice were isolated, embedded in paraffin, and cut into 5 μm sections. Every tenth section was collected and stained with H&E or LFB counterstained with periodic acid Schiff (PAS) as in a previous report [24]. From H&E staining, the number of infiltrating inflammatory cells in one whole spinal cord slide was counted (“total cell number” minus “mean cell number in normal control”) and all of the successive sections were evaluated for each EAE mouse. From LFB staining, both the area of demyelinated white matter and total white matter (WM) in one whole spinal cord slide were measured and all successive sections were evaluated for each EAE mouse, then the demyelinated:total WM ratio was calculated. Independent readers blindly scored the H&E and LFB sections, and at least 3 mice from the different treatment groups were included.
The brain tissues of MS patients were stained with LFB to show the demyelinated area.
Statistical Analysis
A two-tailed Student’s t test was applied for statistical comparison of 2 groups. The EAE score was analyzed using the non-parametric Mann–Whitney U test. The data are presented as mean ± SD unless otherwise indicated, and “mean ± SD” generally represents biological replicates. P < 0.05 was considered statistically significant.
Results
Expression Pattern of miR-30a in Microglia of MS Patients
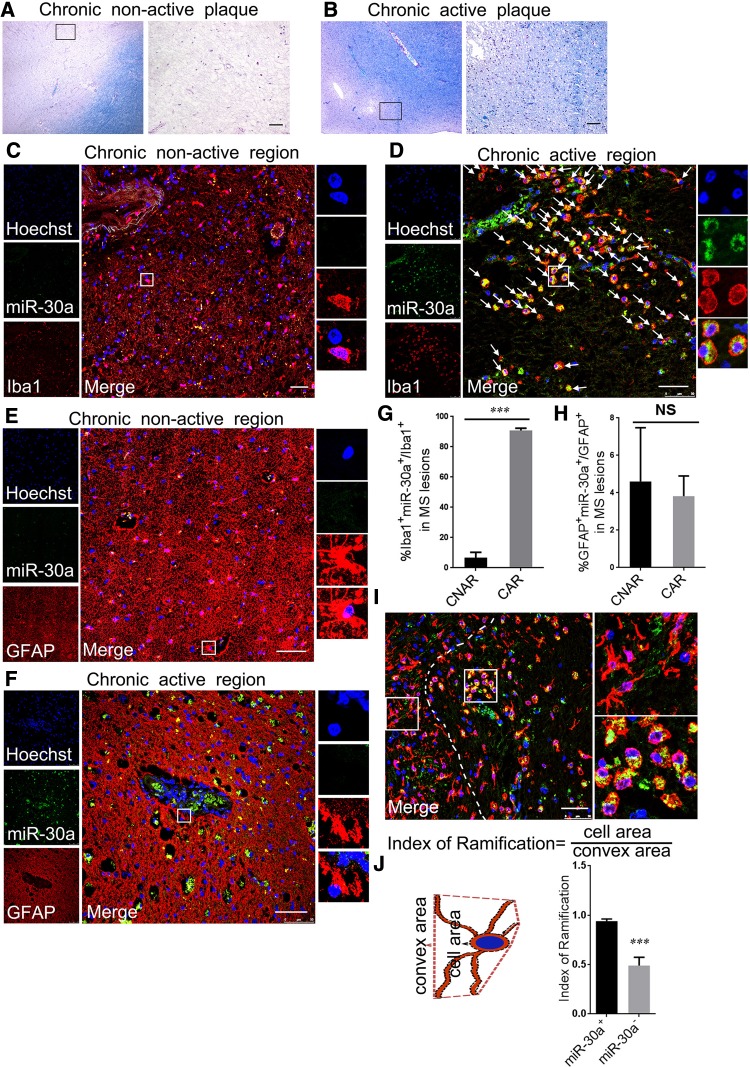
In order to assess the expression of miR-30a in MS lesions, we obtained brain tissues of different MS lesion types from the NBB (Table 1). Using FISH combined with IHF, the expression of miR-30a in microglia and astrocytes, the two primary cells in CNS inflammation, was assessed in both chronic active and chronic inactive lesions. The results showed that miR-30a was highly expressed in chronic active lesions rather than in inactive lesions, mainly in microglia but rarely in astrocytes (Fig. 1A–H). Besides, the expression of miR-30a in microglia was heterogeneous: more was present in microglia with a hypertrophic, jellyfish-like shape than those with a branched shape (Fig. 1I, J). It has been reported that microglia with a jellyfish-like shape are more phagocytic, and tend to be pro-inflammatory, while branched microglia tend to be resting or anti-inflammatory [25]. The differential expression pattern of miR-30a in MS lesions suggested that it may be involved in the inflammatory process by influencing the function of microglia.
Table 1.
Characteristics of MS patients and controls.
| NBB | Autopsy | Sex | Age | Diagnosis | Specific |
|---|---|---|---|---|---|
| 1997-160 | S97/385 | f | 40 | Multiple sclerosis | #5 2,2,2,4,4 |
| 1997-160 | S97/385 | f | 40 | Multiple sclerosis | #6 2,3 |
| 2004-007 | S04/029 | f | 50 | Multiple sclerosis | #2 chron.act. |
| 2004-007 | S04/029 | f | 50 | Multiple sclerosis | #5 chron.inact. |
| 2009-103 | S09/317 | f | 59 | Multiple sclerosis | #1 3(3),4,6 |
| 2006-027 | S06/082 | f | 77 | Multiple sclerosis | #1 4,6 |
| 2006-054 | S06/178 | m | 66 | Multiple sclerosis | #3 active |
| 2006-054 | S06/178 | m | 66 | Multiple sclerosis | #5 chron.inact. |
| 2009-034 | S09/104 | m | 45 | Multiple sclerosis | #5 3(2),4,6 |
| 2012-084 | S12/084 | m | 63 | Multiple sclerosis | #1 1,3(1),4,5I,5II,5II |
| 2007-057 | S07/216 | m | 71 | Multiple sclerosis | #2 4,6,6 |
| 1994-119 | S94/325 | f | 51 | Non-demented control | |
| 1994-123 | S94/338 | f | 76 | Non-demented control | |
| 2012-071 | S12/071 | f | 57 | Non-demented control | |
| 1996-032 | 96/052. | f | 60 | Non-demented control | |
| 1995-092 | S95/258 | f | 63 | Non-demented control | |
| 2012-049 | S12/049 | f | 70 | Non-demented control | |
| 1995-011 | *95/026 | m | 62 | Non-demented control | |
| 1995-084 | S95/240 | m | 72 | Non-demented control | |
| 2011-069 | S11/069 | m | 49 | Non-demented control | |
| 2000-090 | S00/185 | m | 70 | Non-demented control | |
| 1995-084 | S95/240 | m | 72 | Non-demented control |
Annotation for staging and characterization of MS lesions from NBB: #1: Reactive (groups of HLA-positive microglial cells, no demyelination); #2: Active lesion (demyelination, HLA positive microglial cells throughout the lesion); #3: Chronic active lesion (hypocelular gliotic centre, active border with HLA-positive macrophages/microglial cells); Sub score 1–3 reflects degree of activation of HLA positive microglial cells: ramified appearance = 1 up to big rounded foamy morphology = 3; #4: Gliotic inactive hypocellular lesion, with some HLA-positive microgliosis; #5: Grey matter lesion (for cortex not for spinal cord); 6 Shadow plaque.
Fig. 1.
MiR-30a is dynamically expressed in MS lesions. A, B MS lesions characterized using Luxol fast blue-periodic acid Schiff (LFB-PAS). Chronic non-active plaques (A) were extensively demyelinated with few or no inflammatory cells. Chronic active plaques (B) were extensively demyelinated with a rim of inflammatory cells. C–F Representative confocal microscopic images of miR-30a in a chronic non-active lesion (C, E) and a chronic active lesion (D, F) using FISH (LNA probe, green) combined with IHF staining for microglia (Iba1, red) or astrocytes (GFAP, red). Arrows in D indicate miR-30a+Iba1+ cells. G, H Statistical analysis of the proportion of miR-30a+ cells in microglia (G) and astrocytes (H) in MS lesions (n ≥ 6). I A representative image showing high miR-30a (green) expression in jellyfish-like microglia/macrophages (red) in a chronic active MS lesion. J Statistical analysis of the ramification index of miR-30a+ and miR-30a− microglia/macrophages (n ≥ 20). Scale bars, 50 μm; ***P < 0.005 (Student’s t test), mean ± SD.
Expression Pattern of miR-30a in Microglia of EAE Mice
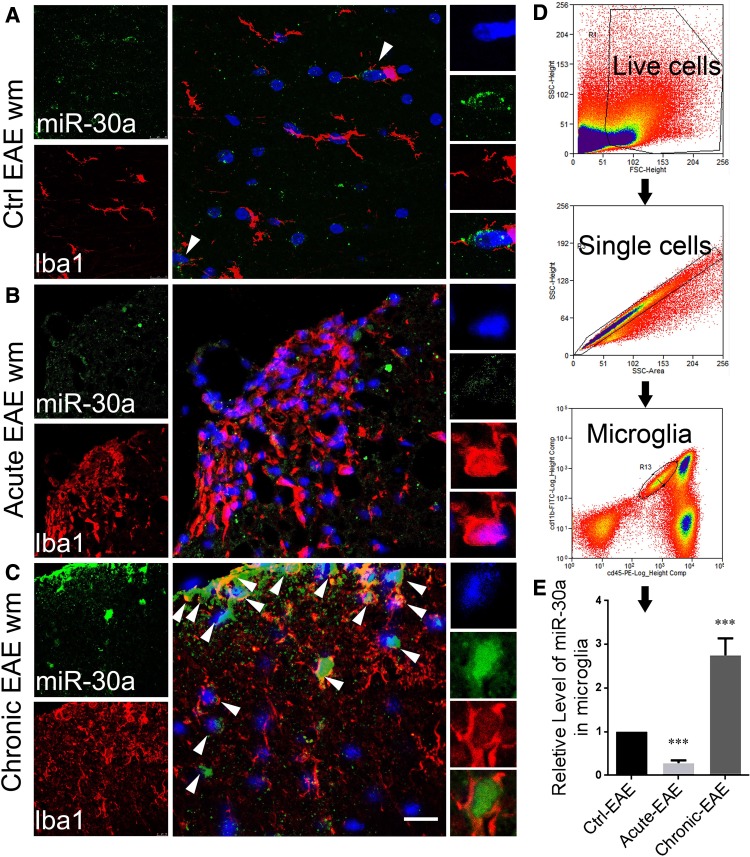
To study the expression pattern of miR-30a in a well-defined developing process, MOG (myelin oligodendrocyte glycoprotein)-EAE, a classical animal model of MS, was established. The spinal cords of EAE mice were analyzed by FISH-IHF staining 15 days after induction in the acute inflammatory phase, and 40 days after induction in the chronic inflammatory phase. The results showed that the expression of miR-30a in microglia in the chronic phase was significantly higher than that of control (Fig. 2A–C). Furthermore, we sorted microglia from the brains and spinal cords of EAE mice by flow cytometry and analyzed them by qPCR (Fig. 2D). The results also showed higher expression of miR-30a in EAE mice in the chronic phase than that of control (Fig. 2E). These results indicated that miR-30a tends to be highly expressed in inactivated microglia in the chronic phase. These are usually reported to be pro-inflammatory [13], indicating a positive correlation between the expression level of miR-30a and the inflammatory state of microglia in EAE.
Fig. 2.
MiR-30a is dynamically expressed in the microglia of mice with EAE. A–C Representative confocal microscopic images of miR-30a in spinal sections from the white matter (WM) of CFA-immunized (control) (A), acute EAE (16 dpi) (B), and chronic EAE (40 dpi) (C) mice (scale bar 20 μm). Arrowheads in A and C indicate miR-30a+ Iba1+ cells. D Schematic of microglia isolation. E Quantitative PCR analysis of miR-30a in microglia at different stages of EAE (n = 6, ***P < 0.005, Student’s t test, mean ± SD).
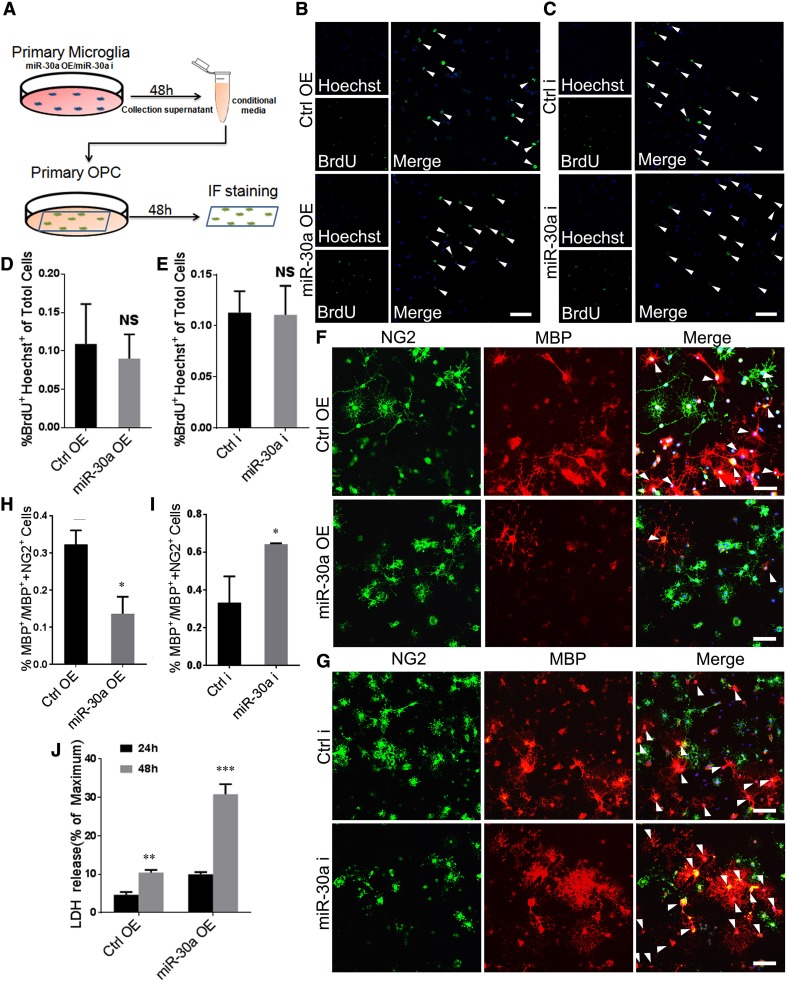
MiR-30a-Modified Microglia Regulate the Survival and Differentiation of Oligodendrocyte Precursor Cells (OPCs) In Vitro
Oligodendrocytes are the main cells involved in MS and they are replenished by OPCs. To investigate whether high expression of miR-30a in microglia affects the survival and differentiation of co-cultured OPCs, we used conditioned medium from miR-30a-modified microglia (Figs. 3A, S1). BrdU incorporation showed no significant difference between groups, indicating no influence on the proliferation of OPCs (Fig. 3B–E). We also assessed the differentiation of co-cultured OPCs by anti-MBP immunofluorescence, which showed that the MGmiR-30aOE (microglia overexpressing miR-30a) supernatant suppressed OPC differentiation, whereas the MGmiR-30ai (microglia with miR-30a interference) supernatant promoted it (Fig. 3F–I). Moreover, the level of LDH released by apoptotic or necrotic OPCs in the supernatant was higher when they were co-cultured with the conditioned medium from MGmiR-30aOE (Fig. 3J). These in vitro results indicated that high expression of miR-30a in microglia causes damage to OPCs by promoting their apoptosis and inhibiting their differentiation.
Fig. 3.
MiR-30a-modified microglia affect OPC survival and differentiation. A Schematic of the co-culture process. B, C BrdU incorporation assay of OPCs incubated with conditioned medium from MGmiR-30aOE or MGmiR-30ai for 6 h versus control (scale bars 50 µm). Arrowheads in B and C indicate BrdU+ cells. D, E Statistical analysis of BrdU+/Hoechst+ cells between groups in B and C. F, G Anti-NG2 (green) and anti-MBP (red) immunocytofluorescence of OPCs incubated with conditioned medium from MGmiR-30aOE (F) and MGmiR-30ai (G) for 72 h versus control (scale bars 50 µm). Arrowheads in F and G indicate MBP+ cells. H, I Statistical analysis of MBP+/(NG2+ or MBP+) cells between groups in F and G (n = 3). J LDH assay in culture supernatants of OPCs incubated with conditioned medium from MGmiR-30aOE for 24 h or 48 h versus control (n = 3). *P < 0.05, **P < 0.01, ***P < 0.005 versus control (Student’s t test); mean ± SD.
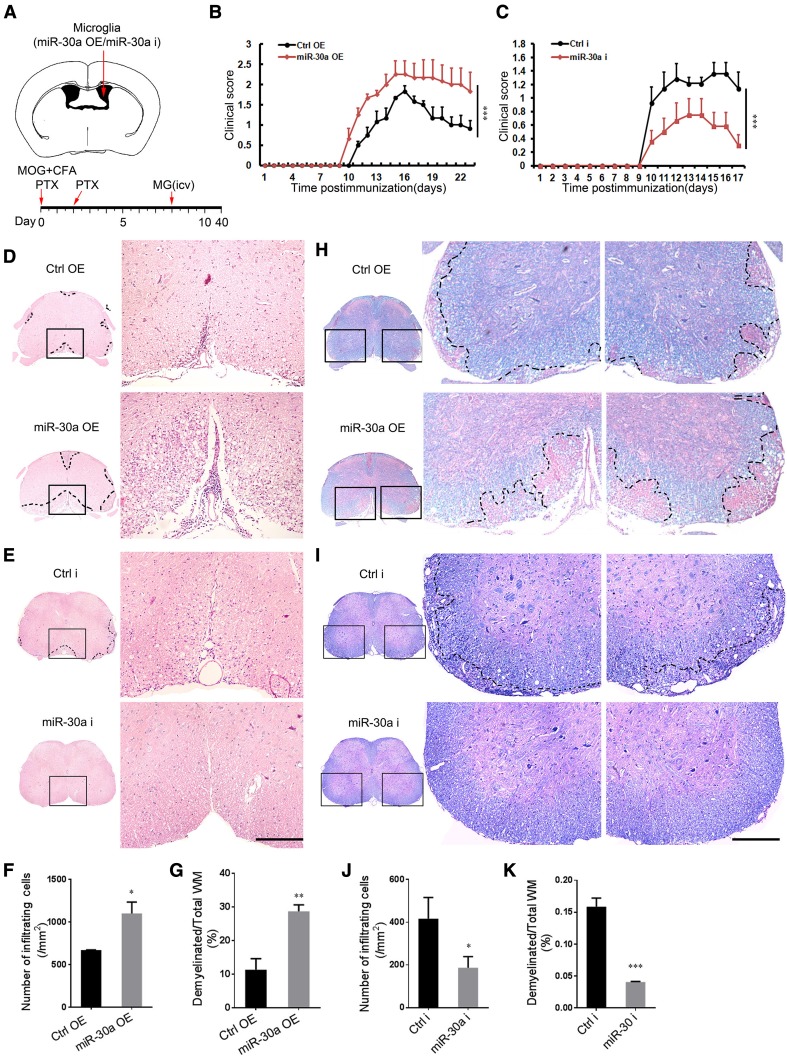
MiR-30a-Modified Microglia Regulate EAE Progression
To elucidate the role of miR-30a in microglial functions in vivo, we transplanted miR-30a-modified microglia into the cerebral ventricles of EAE mice at 8 dpi (Fig. 4A). The clinical symptoms of EAE mice were evaluated daily and the spinal cords were isolated for further histological analysis. The scores showed that the transplantation of MGmiR-30aOE exacerbated EAE (Fig. 4B), whereas MGmiR-30ai alleviated it (Fig. 4C). H&E staining showed that the infiltration of inflammatory cells into the lumbar spinal cord of the EAE mice at 20 dpi was increased after MGmiR-30aOE transplantation (Fig. 4D, F), and reduced after MGmiR-30ai transplantation (Fig. 4E, J). In accordance with the in vitro results, the anti-PCNA IHF and TUNEL analysis showed that the survival and proliferation of OPCs were not affected (Fig. S2). However, LFB staining showed exacerbated myelin loss in the white matter of the EAE mice at 20 dpi after MGmiR-30aOE transplantation (Fig. 4H, G) and attenuated myelin loss after MGmiR-30ai transplantation (Fig. 4I, K). These results showed that microglia with high expression of miR-30a aggravate EAE.
Fig. 4.
MiR-30a-modified microglia regulate the progression of EAE. A Schematic of the injection site and experimental timeline. B, C Clinical scores of EAE mice transplanted with MGCtrlOE/MGmiR-30aOE B or MGCtrli/MGmiR-30ai C (n = 8 mice). D, E, H, I Representative spinal cord sections from EAE mice after H&E staining (D, E) and Luxol fast blue staining (H, I) (scale bars 200 µm). F, G, J, K Quantification of infiltrating cells in the white matter (WM) (F, J) in D and E and the percentage of demyelinated WM in total WM (G, K) in H and I. Data from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus control (B, C Mann–Whitney U test; F, G, J, K Student’s t test); mean ± SEM (B, C) or mean ± SD (H–K).
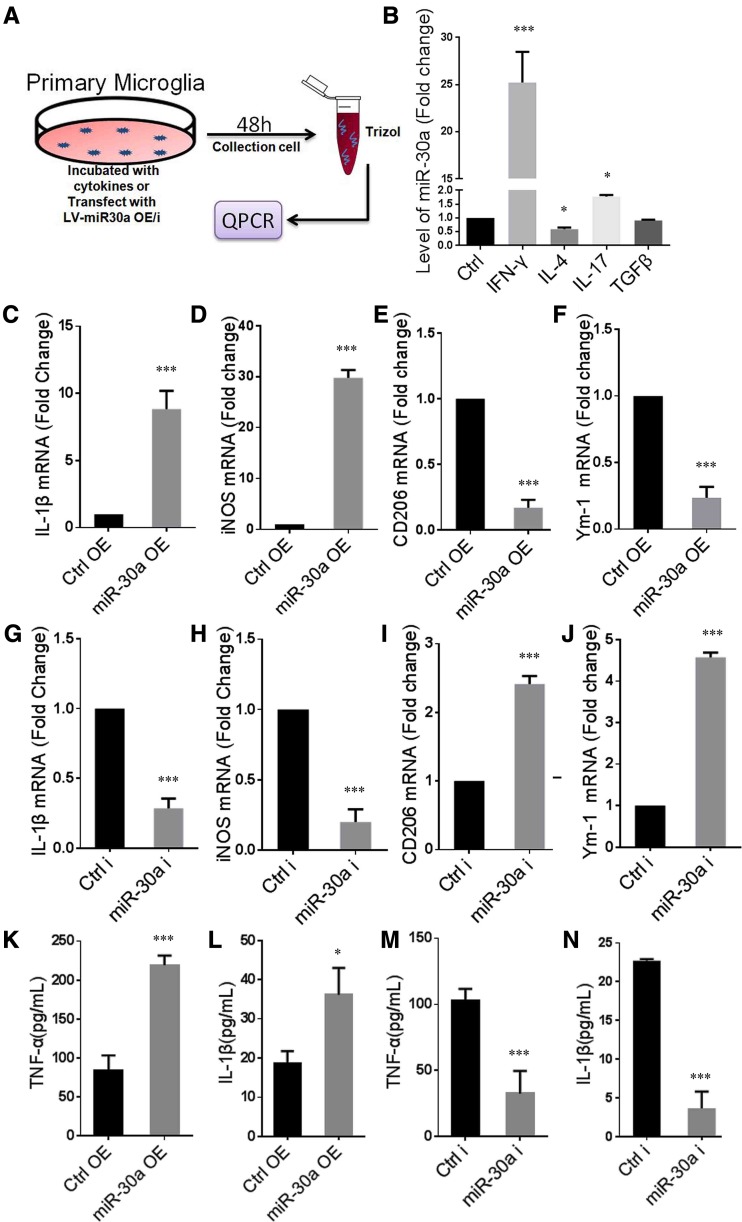
MiR-30a Promotes Microglial Inflammatory Response
To explore the effect of miR-30a on microglial inflammatory responses, we further studied its expression and function in vitro (Fig. 5A). First, we induced primary cultured microglia into different inflammatory states by adding different cytokines and found that more miR-30a was expressed in IFN-γ-stimulated microglia and less in IL-4-stimulated cells. The results showed more miR-30a was expressed in pro-inflammatory microglia (Fig. 5B). More importantly, microglia overexpressing miR-30a increased the expression of IL-1β and iNOS, which are pro-inflammatory, and decreased the expression of Ym-1 and CD206, which are anti-inflammatory and reparative (Fig. 5C–F). Conversely, interference with miR-30a in microglia resulted in a decrease in the expression of IL-1β and iNOS and an increase in the expression of Ym-1 and CD206 (Fig. 5G–J). ELISA showed similar changes in TNF-α and IL-1β protein expression in the supernatant from microglia overexpressing miR-30a (Fig. 5K, L) or microglia with miR-30a interference (Fig. 5M, N). Besides, miR-30a did not affect the apoptosis of microglia (Fig. S3). These results indicated that miR-30a directly promotes the inflammatory response of microglia and suppresses their anti-inflammatory and reparative effects, which aggravate EAE.
Fig. 5.
MiR-30a promotes microglial inflammatory response. A Schematic of the protocol using primary cultured microglia. B Quantitative PCR analysis of miR-30a in microglia treated with different cytokines for 48 h versus untreated control (n = 3). C–J Quantitative PCR analysis of pro-inflammatory cytokines IL-1β (C, G) and iNOS (D, H), and anti-inflammatory markers CD206 (E, I) and Ym-1 (F, J) in microglia transduced with miR-30aOE (C–F) or miR-30ai (G–J) lentivirus vectors versus control (n = 3). K–N ELISA analysis of TNF-α (K, L) and IL-1β (M, N) in culture supernatants of microglia transduced with miR-30aOE (K, L) or miR-30ai (M, N) lentivirus vectors versus control (n ≥ 3 experiments). *P < 0.05, ***P < 0.005 versus control (Student’s t test); B one-way ANOVA; C–N, Student’s t test); mean ± SEM (B) or mean ± SD (C–N).
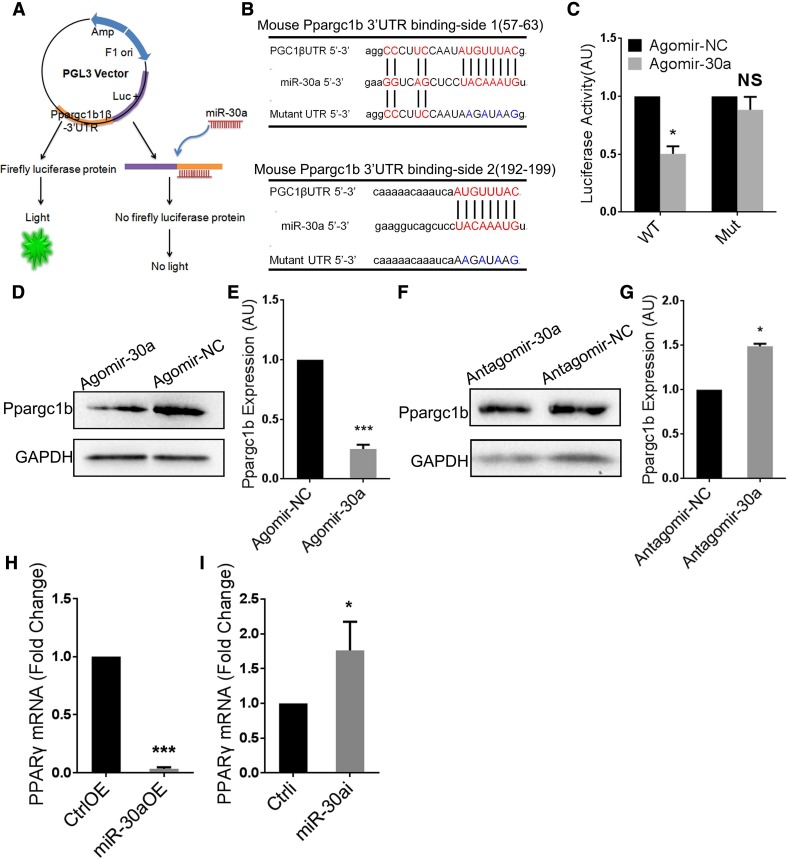
MiR-30a Aggravates Microglial Inflammatory Response by Targeting PPARγ Coactivator-1 beta (Ppargc1b)
To elucidate how miR-30a aggravates the microglial inflammatory response, we used Targetscan and miRBase to search for potential target genes, and the 3′ UTR region of Ppargc1b was predicted to contain four complementary binding sites for miR-30a. Ppargc1b is a co-activator of PPARγ, which is one of the most important transcription factors in the microglial anti-inflammatory response [26–28]. We verified the binding using a luciferase reporter system (Fig. 6A, B). Co-transfecting 293T cells with miR-30a/Ppargc1b 3′ UTR inhibited luciferase activity, whereas co-transfection of miR-30a/mutant Ppargc1b 3′ UTR had no effect (Fig. 6C). The results indicated site-specific binding between miR-30a and the 3′ UTR of Ppargc1b. Furthermore, western blots showed down-regulated expression of Ppargc1b in microglia overexpressing miR-30a (Fig. 6D, E), while interfering miR-30a had the opposite effect (Fig. 6F, G). Besides, qPCR showed that the expression of PPARγ decreased in microglia overexpressing miR-30a (Fig. 6H), while interfering with miR-30a increased it (Fig. 6I). These results validated regulation of the expression of Ppargc1b by miR-30a. Considering the importance of Ppargc1b in PPARγ activation and expression, miR-30a may aggravate the inflammatory response of microglia by inhibiting the PPARγ signal transduction pathway, thus exacerbating EAE.
Fig. 6.
Ppargc1b is a functional target of miR-30a in microglia. A Schematic of the luciferase reporter system. B Putative miR-30a binding sites and mutations in Ppargc1b mRNA 3′ UTR. C Luciferase activity of reporter carrying the wild-type (WT) or mutated (Mut) Ppargc1b mRNA 3′ UTR co-transfected into HEK293T cells with Agomir-30a or Agomir-NC. D, F Western blots of Ppargc1b in microglia transduced with miR-30aOE (D) or miR-30ai (F) lentivirus vector. E, G Statistical analysis of Ppargc1b densitometry normalized to GAPDH in D and F (n = 3). H, I Quantitative PCR analysis of PPARγ in microglia transduced with miR-30aOE (H) or miR-30ai (I) lentivirus vector versus control (n = 3). *P < 0.05 (Student’s t test); mean ± SD.
Discussion
MS is an autoimmune as well as a neurodegenerative disease [10]. The major resident immune cells, microglia, play a crucial role in the inflammatory process of MS and in the disruption of neuronal elements within the CNS [6, 29]. MicroRNAs are a class of post-transcriptional regulatory molecules, some of which have been reported to be important regulators of microglial function, such as miR-124 and miR-155 [17, 18, 30, 31]. In this work, we focused on microRNAs expressed in the chronic lesions of MS patients and identified miR-30a as functional in microglia. Studies showed that microglia overexpressing miR-30a promoted the apoptosis of OPCs, inhibited the differentiation of OPCs, and aggravated EAE. Our work demonstrated that miR-30a is a new regulator of microglial function, and a likely participant in the pathogenesis of MS.
MiR-30a has been found to play functional roles in biological processes like tumorigenesis and autophagy [21, 32, 33]. Remarkably, two reports have shown that miR-30a is involved in the differentiation of Th17 cells in MS [20, 22]. Although miR-30a was shown to play an anti-inflammatory role by inhibiting the differentiation of Th17 cells in these reports, our work showed that miR-30a is a pro-inflammatory factor in microglia, indicating complicated roles of miR-30a in different cell types. The spatial distribution of miR-30a in brain slices from MS patients, high in microglia versus rare in astrocytes, and high in chronic lesions versus low in normal regions, also demonstrated a distinctive role in MS. Previous work has shown that intravenous injection of lentivirus encoding a pre-miR-30a sequence into EAE mice prevents the disease from developing fully [20, 22]. However, transplantation of microglia overexpressing miR-30a into the cerebral ventricles of EAE mice aggravated the disease in our study, indicating the need for a comprehensive consideration of the use of miR-30a as a therapeutic target for inflammatory diseases like MS. Cell type-specific administration of miR-30a, like promoter-specific lentivirus, may be an appropriate approach. Besides, the five members of the miR-30 family share the same 8-nucleotide seed sequence, so whether they work in a similar way deserves further study.
To investigate how miR-30a regulates the microglial inflammatory response, we used target gene prediction software and found that miR-30a could bind to Ppargc1b at four binding sites. Ppargc1b is a necessary co-activator for the functional performance of PPARγ, which is one of the core transcription factors modulating microglia in an anti-inflammatory direction [14, 27]. High expression of miR-30a decreased the Ppargc1b protein level and reduced PPARγ activity and expression, inducing microglia to be pro-inflammatory and cytotoxic. Pro-inflammatory cytokines like IL-1β secreted by microglia damaged OPCs, and IL-1β has been reported to inhibit their proliferation and differentiation [34]. Remarkably, miR-30a targets not only Ppargc1b but also many other molecules, which makes it possible that other pathways could regulate the microglial inflammatory response to miR-30a.
Our work demonstrates that miR-30a is a novel regulator of the microglial inflammatory response, and that miR-30a-modified microglia regulate the progression of EAE.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Netherlands Brain Bank for kindly providing the brain slices from MS patients. This work was supported by the International Cooperation and Exchange of the National Natural Science Foundation of China (81461138035), the National Natural Science Foundation of China (81371326, 31371068, and 31571066), and the National Key Research and Development Program of China (2016YFA0100802).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0153-y) contains supplementary material, which is available to authorized users.
Xue Fang, Dingya Sun, and Zhihong Wang have contributed equally to this work.
Contributor Information
Cheng He, Email: chenghe@smmu.edu.cn.
Li Cao, Email: caoli@smmu.edu.cn.
References
- 1.Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J Autoimmun. 2014;48–49:134–142. doi: 10.1016/j.jaut.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8:647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 3.Ciccarelli O, Thompson A. Multiple sclerosis in 2015: managing the complexity of multiple sclerosis. Nat Rev Neurol. 2016;12:70–72. doi: 10.1038/nrneurol.2016.2. [DOI] [PubMed] [Google Scholar]
- 4.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 5.Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015;14:406–419. doi: 10.1016/S1474-4422(14)70305-9. [DOI] [PubMed] [Google Scholar]
- 6.Cao L, He C. Polarization of macrophages and microglia in inflammatory demyelination. Neurosci Bull. 2013;29:189–198. doi: 10.1007/s12264-013-1324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogie JF, Stinissen P, Hendriks JJ. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 2014;128:191–213. doi: 10.1007/s00401-014-1310-2. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, He H, Yang Z, Zhu G, Kang L, Jing X, et al. Programmed death ligand-1 on microglia regulates Th1 differentiation via nitric oxide in experimental autoimmune encephalomyelitis. Neurosci Bull. 2016;32:70–82. doi: 10.1007/s12264-015-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 10.Stys PK, Zamponi GW, van Minnen J, Geurts JJ. Will the real multiple sclerosis please stand up? Nat Rev Neurosci. 2012;13:507–514. doi: 10.1038/nrn3275. [DOI] [PubMed] [Google Scholar]
- 11.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 12.Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Ding F, Gu X. Non-coding RNAs as emerging regulators of neural injury responses and regeneration. Neurosci Bull. 2016;32:253–264. doi: 10.1007/s12264-016-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso AL, Guedes JR, Pereira de Almeida L, Pedroso de Lima MC. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology 2012, 135: 73–88. [DOI] [PMC free article] [PubMed]
- 19.O’Brien JH, Hernandez-Lagunas L, Artinger KB, Ford HL. MicroRNA-30a regulates zebrafish myogenesis through targeting the transcription factor Six1. J Cell Sci. 2014;127:2291–2301. doi: 10.1242/jcs.143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu X, Zhou J, Wang T, Han J, Ma L, Yu H, et al. MiR-30a inhibits Th17 differentiation and demyelination of EAE mice by targeting the IL-21R. Brain Behav Immun. 2016;57:193–199. doi: 10.1016/j.bbi.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Zhang N, Wang X, Huo Q, Sun M, Cai C, Liu Z, et al. MicroRNA-30a suppresses breast tumor growth and metastasis by targeting metadherin. Oncogene. 2014;33:3119–3128. doi: 10.1038/onc.2013.286. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Sun D, Guan Y, Wang Z, Sang D, Liu M, et al. Disulfiram and diphenhydramine hydrochloride upregulate miR-30a to suppress IL-17-associated autoimmune inflammation. J Neurosci. 2016;36:9253–9266. doi: 10.1523/JNEUROSCI.4587-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Planell-Saguer M, Rodicio MC, Mourelatos Z. Rapid in situ codetection of noncoding RNAs and proteins in cells and formalin-fixed paraffin-embedded tissue sections without protease treatment. Nat Protoc. 2010;5:1061–1073. doi: 10.1038/nprot.2010.62. [DOI] [PubMed] [Google Scholar]
- 24.Yu Z, Sun D, Feng J, Tan W, Fang X, Zhao M, et al. MSX3 switches microglia polarization and protects from inflammation-induced demyelination. J Neurosci. 2015;35:6350–6365. doi: 10.1523/JNEUROSCI.2468-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, et al. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010;11:503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP. PPARgamma and the innate immune system mediate the resolution of inflammation. PPAR Res. 2015;2015:549691. doi: 10.1155/2015/549691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juhas U, Ryba-Stanislawowska M, Szargiej P, Mysliwska J. Different pathways of macrophage activation and polarization. Postepy Hig Med Dosw (Online) 2015;69:496–502. doi: 10.5604/17322693.1150133. [DOI] [PubMed] [Google Scholar]
- 29.Sanders P, De Keyser J. Janus faces of microglia in multiple sclerosis. Brain Res Rev. 2007;54:274–285. doi: 10.1016/j.brainresrev.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Moore CS, Rao VT, Durafourt BA, Bedell BJ, Ludwin SK, Bar–Or A, et al. miR–155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann Neurol. 2013;74:709–720. doi: 10.1002/ana.23967. [DOI] [PubMed] [Google Scholar]
- 31.Ponomarev ED, Veremeyko T, Weiner HL. MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia. 2013;61:91–103. doi: 10.1002/glia.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu Y, Cao L, Yang L, Kang R, Lotze M, Tang D. microRNA 30A promotes autophagy in response to cancer therapy. Autophagy. 2012;8:853–855. doi: 10.4161/auto.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen X, et al. MicroRNA-30a sensitizes tumor cells to cis-platinum via suppressing beclin 1-mediated autophagy. J Biol Chem. 2012;287:4148–4156. doi: 10.1074/jbc.M111.307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie D, Shen F, He S, Chen M, Han Q, Fang M, et al. IL-1beta induces hypomyelination in the periventricular white matter through inhibition of oligodendrocyte progenitor cell maturation via FYN/MEK/ERK signaling pathway in septic neonatal rats. Glia. 2016;64:583–602. doi: 10.1002/glia.22950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.