Scheme 1.
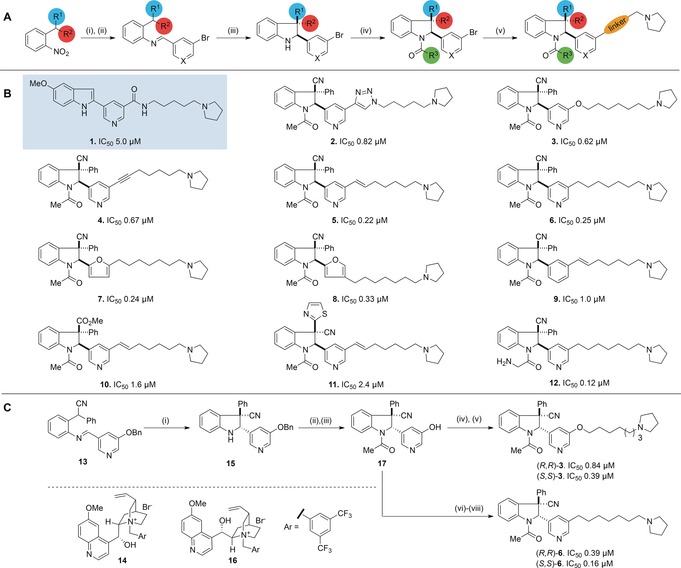
A) General synthetic strategy for racemic synthesis of KDM2A inhibitors. Reagents and conditions: i) Zn powder (10 equiv), NH4Cl (15 equiv), 5:1 acetone/H2O or H2, Pd/C (10 % w/w), EtOAc; ii) ArCHO (1.3 equiv), MgSO4 (5 equiv), PhMe or ArCHO (1.3 equiv), pyrrolidine (0.1 equiv), 3 Å sieves, CH2Cl2; iii) KOtBu (1.1 equiv), PhMe, 0 °C; iv) R3COCl (2–5 equiv), pyridine (2–5 equiv), CH2Cl2; v) metal‐catalysed cross‐coupling. B) Key structure–activity relationships. IC50 values were determined by RapidFire MS and confirmed by AlphaScreen. All compounds are racemic. C) Catalytic enantioselective synthesis. Reagents and conditions: i) CsOH⋅H2O (2.0 equiv), catalyst 14/16 (10 mol %), PhMe, −30 °C. Catalyst 14: 89 %, d.r. 10:1, e.r. 88:12; catalyst 16: 84 %, d.r. 7:1, e.r. 17:83; ii) CH3COCl (2.0 equiv), pyridine (2.0 equiv), CH2Cl2; iii) H2, Pd/C (10 % w/w), 88 % (2 steps); iv) K2CO3 (5 equiv), Br(CH2)7Br (4 equiv), acetone, reflux, 38 %; v) K2CO3 (5 equiv) pyrrolidine (4 equiv), CH3CN, 65 %; vi) PhN(SO2CF3)2 (1.1 equiv), DIPEA (2.0 equiv), CH2Cl2, 0 °C, 85 %; vii) 7‐pyrrolidine‐hept‐1‐yne (1.5 equiv), PdCl2(PPh3)2 (5 mol %), CuI (5 mol %), HN(iPr)2, 75 °C, 79 %; viii) H2, Pd/C (10 % w/w), MeOH, 74 %. DIPEA=(iPr)2NEt.
