Fig. 1.
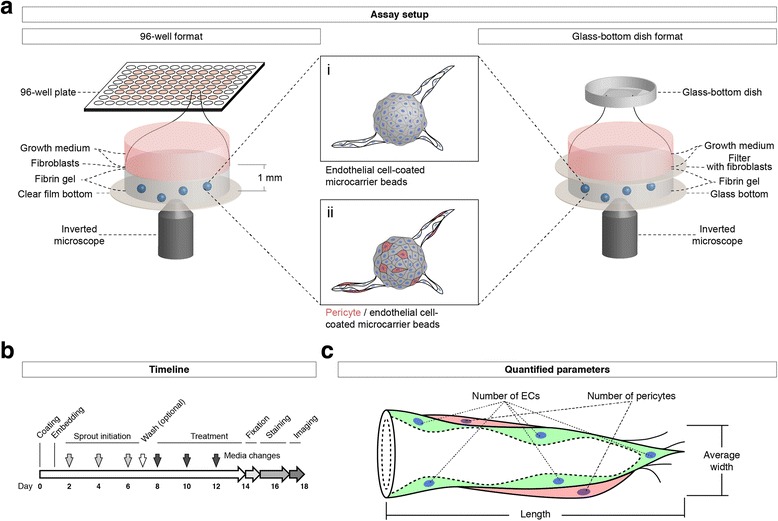
Setup and workflow of the EC/pericyte sprouting assay. a Schematic drawing of the assay setup: A 1:10 co-culture of pericytes (red) and endothelial cells are coated on microcarrier beads (centre) that are then embedded into fibrin gels in each of the inner 60 wells of a 96-well plate (left). Alternatively, cell-coated beads are embedded into fibrin gels in a glass-bottom culture dish (right). The fibrin gels are subsequently covered with a layer of fibroblast cells (left), which can also be placed onto filters (right) to facilitate removal and subsequent staining. Developing sprouts are cultured in growth medium containing the desired treatment, and they can be imaged using an inverted microscope. b Timeline of the assay procedure: after coating ECs or ECs/pericytes on microcarrier beads and embedding them in fibrin gels, sprouts are grown for up to 6 days, optionally supplied with 10-μM vanadate to accelerate sprout growth; after removal of vanadate by washing with growth medium, treatment with test compounds or growth factors can be pursued for another 6 days; for image analysis, sprouts are fixed, stained, and imaged using an inverted microscope. c Schematic representation of a sprout containing ECs (green) and pericytes (red), indicating the quantified sprout parameters
