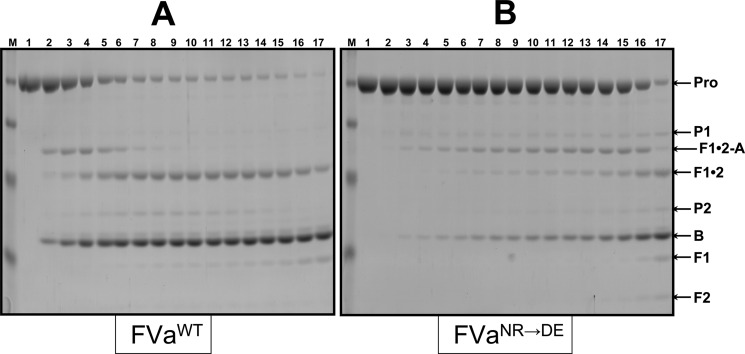
Figure 3.
Analysis of the activation of plasma-derived hPro by prothrombinase. Plasma-derived hPro (1.4 μM) was incubated in different mixtures with PCPS vesicles (20 μM), and prothrombinase was assembled with either wild-type rhfVa (panel A, 20 nM) or rhfVaNR→DE (panel B, 20 nM), as described in the Experimental Section. At selected time intervals, aliquots of the reactions were withdrawn and treated as described in the Experimental Section. M represents the lane with the molecular weight markers (from top to bottom): Mr 98 000, Mr 64 000, Mr 50 000, Mr 36 000, Mr 22 000. Lanes 1–17 represent samples from the reaction mixture before (0 min) the addition of fXa and 20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, 240 s, 5, 6, 10, and 20 min, respectively, following the addition of fXa. The hPro-derived fragments are shown as follows: Pro (hPro, amino acid residues 1–579); P1 (prethrombin-1, amino acid residues 156–579); F1•2-A (fragment 1•2-A chain, amino acid residues 1–320); F1•2 (fragment 1•2, amino acid residues 1–271); P2 (prethrombin-2, amino acid residues 272–579); B (B chain of α-thrombin, amino acid residues 321–579). F1 (fragment 1, amino acid residues 1−155); F2 (fragment 2, amino acid residues 156−271).