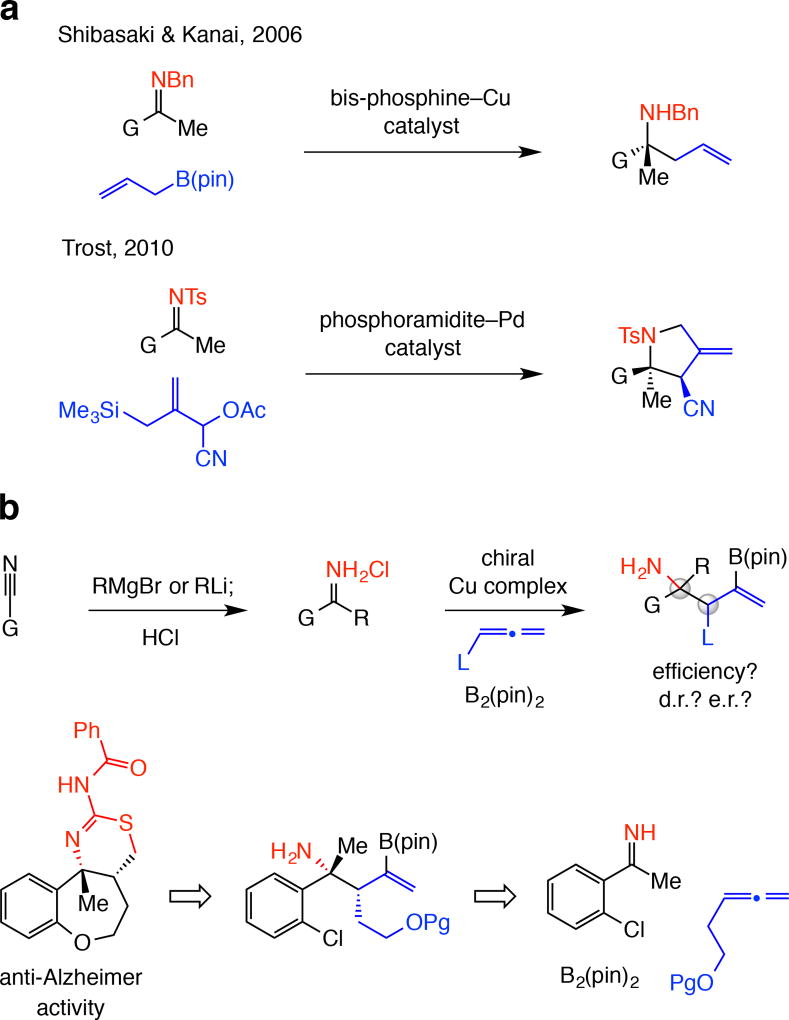
Figure 1. State-of-the-art in allyl additions to ketimines and goals of this study.
There are significant exisiting limitations and a number of compelling issues remain unaddressed. a, There are only a small number of methods for catalytic enantioselective addition of an allyl group to a ketimine. The substrate is typically equipped with an activating/protecting group, which might prove difficult to remove in the presence of similar functional groups within a product structure (e.g., another N-benzylamine). b, A direct approach to synthesis of α-tertiary amines may entail preparation of the requisite unprotected N-H ketimine through alkylation of readily available nitriles followed by catalytic site-, diastereo- and enantioselective multicomponent addition of 2-boryl-substituted allyl groups. One application relates to synthesis of the core tricyclic structure of a set of heterocyclic molecules that exhibit strong anti-Alzheimer activity. Bn, benzyl; Ts, tosyl; Ac, acyl; pin, pinacolato; G, R, L, functional groups; Pg, protecting group.