Table 1.
Probing efficiency and diastereoselectivity with different (achiral) catalyst types.
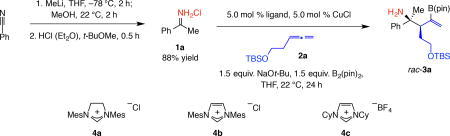
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Ligand | Conv. (%)* | Yield (%)† | d.r.* |
| 1 | PPh3 | 60 | 49 | >98:2 |
| 2 | PCy3 | 71 | 52 | >98:2 |
| 3 | rac-binap | 75 | 72 | >98:2 |
| 4 | 4a | 80 | 66 | >98:2 |
| 5 | 4b | 55 | 53 | >98:2 |
| 6 | 4c | >98 | 90 | >98:2 |
Reactions were carried out under N2 atmosphere; 1.2:1 ratio of ketimine:allene was used.
Conversion (based on allene consumption; (includes desired and decomposition products) and diastereomeric ratio (d.r.) values were measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures; variance of values is estimated to be <±2%.
Yield of isolated and purified product (<±5%). See the Supplementary Information for details).
Abbreviations: TBS, tert-butyldimethylsilyl; pin, pinacolato; Mes, 2,4,6-Me3C6H2; Cy, cyclohexyl; rac, racemic; binap, 2,2’-bis(diphenylphosphino)-1,1’-binaphthalene.
