Table 2.
Studies to identify an effective chiral catalyst.
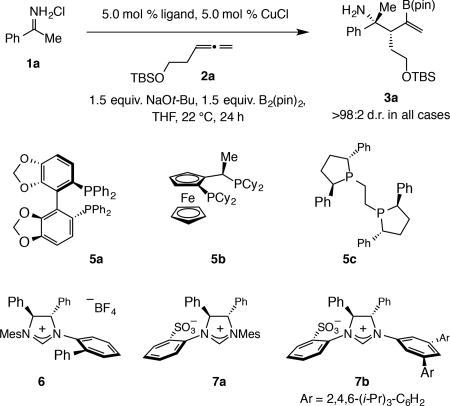
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Ligand | Conv. (%)* | Yield (%)† | e.r.†† |
| 1 | R-binap | 70 | 65 | 44:56 |
| 2 | 5a | 55 | 51 | 18:82 |
| 3 | 5b | 65 | 51 | 19.5:80.5 |
| 4 | 5c | 29 | 27 | 69:31 |
| 5 | 6 | 80 | 51 | 40:60 |
| 6 | 7a | >98 | 65 | 55:45 |
| 7 | 7b | 54 | 51 | 95:5 |
Reactions were carried out under N2 atmosphere; 1.2:1 ratio of ketimine:allene was used.
Conversion (consumption of allene; (includes desired and decomposition products) and d.r. was measured by analysis of 400 MHz 1H NMR spectra of unpurified mixtures; variance of values is estimated to be <±2%.
Yield of isolated and purified products (<±5%).
Enantiomeric ratio (e.r.) values were determined by HPLC analysis (<±1%) (see the Supplementary Information for details).
Abbreviations: TBS, tert-butyldimethyl silyl; pin, pinacolato; Mes, 2,4,6-Me3C6H2; Cy, cyclohexyl; binap, 2,2’-bis(diphenylphosphino)-1,1’-binaphthalene.
