Table 3.
Catalytic diastereo- and enantioselective additions to N-H ketimines.
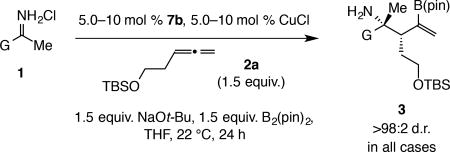
| |||
|---|---|---|---|
|
| |||
| Entry | G | Mol %; Yield (%)† | e.r.†† |
| 1 | C6H5 (a) | 7.5; 76 | 95:5 |
| 2 | o-MeOC6H4 (b) | 10; 95 | 98.5:1.5 |
| 3 | o-ClC6H4 (c) | 7.5; 91 | 99.5:0.5 |
| 4 | o-FC6H4 (d) | 7.5; 72 | 97.5:2.5 |
| 5 | o,o-F2C6H3 (e) | 7.5; 91 | 98:2 |
| 6 | 1-naphthyl (f) | 10; 66 | 98.5:1.5 |
| 7 | o-MeC6H4 (g) | 7.5; 59 | 99.5:0.5 |
| 8 | m-FC6H4 (h) | 5.0; 64 | 94:6 |
| 9 | m-MeOC6H4 (i) | 10; 57 | 98.5:1.5 |
| 10 | 2-naphthyl (j) | 7.5; 81 | 93:7 |
| 11 | p-ClC6H4 (k) | 7.5; 56 | 92.5:7.5 |
| 12 | p-FC6H4 (l) | 5.0; 71 | 95:5 |
| 13 | p-MeOC6H4 (m) | 7.5; 39 | 92.5:7.5 |
| 14 | CyCH2 (n) | 10; 38 | 91:9 |
| 15 | Cy (o) | 10; 48 | 95:5 |
Reactions were carried out under N2 atmosphere; >98% disappearance of ketimine in all cases (includes desired and decomposition products).
Yield of isolated and purified products (<±5%).
Enantiomeric ratios determined by HPLC analysis (<±1%; see the Supplementary Information for details).
Abbreviations: TBS, tert-butyldimethylsilyl; pin, pinacolato; Cy, cyclohexyl.
Experiments were performed at least in triplicate. See the Supplementary Information for details and the results with achiral imidazolinium salt 4c.
