Abstract
Background
Appropriate thromboprophylaxis for patients with atrial fibrillation or atrial flutter (AF) remains a national challenge. The recent availability of direct oral anticoagulants (DOACs) with comparable efficacy and improved safety compared with warfarin alters the balance between risk factors for stroke and benefit of anticoagulation. Our objective was to examine the impact of DOACs as an alternative to warfarin on the net benefit of oral anticoagulant therapy (OAT) in a real-world population of AF patients.
Methods
Retrospective cohort study of patients with paroxysmal or persistent nonvalvular AF. We updated an Atrial Fibrillation Decision Support Tool (AFDST) to include DOACs as treatment options. The tool generates patient–specific recommendations based upon individual patient risk factor profiles for stroke and major bleeding, using quality-adjusted life years (QALYs) calculated for each treatment strategy by a decision analytic model. The setting included inpatient and ambulatory sites in an academic health center in the mid-western United States. Study involved 5,121 adults with nonvalvular AF seen for any ambulatory visit or inpatient hospitalization over the 1-year period (January through December 2016). Outcome measure was net clinical benefit in QALYs.
Results
When DOACs are a therapeutic option, the AFDST recommends OAT for 4,134 (81%) patients, and no antithrombotic therapy or aspirin for 489 (9%). A strong recommendation for OAT could not be made in 498 (10%) patients. When warfarin is the only option, OAT is recommended for 3,228 (63%) patients, and no antithrombotic therapy or aspirin for 973 (19%). A strong recommendation for OAT could not be made in 920 (18%) patients. In total, 1,508 QALYs could be gained were treatment changed to that recommended by the AFDST.
Conclusions
Availability of DOACs increases the proportion of patients for whom oral anticoagulation therapy is recommended in a real-world cohort of AF patients and increased projected QALYs by more than 1,500 when all patients are receiving thromboprophylaxis as recommended by the AFDST compared with current treatment.
Introduction
The stroke-related cost of underuse of AF thromboprophylaxis is over $8 billion.(1) Yet there continues to be widespread underutilization, or, at times, inappropriate use of thromboprophylaxis. Partly responsible is the complex interplay of treatments, risks and benefits along with variability in patient adherence and health literacy. The decision has been further complicated by the introduction of direct oral anticoagulants (DOACs). In 2011, we posited that a new generation of anticoagulants with improved safety and similar, if not improved, efficacy compared with warfarin, would change the “tipping point” in the balance between risk and benefit for anticoagulation therapy.(2) By tipping point, we mean the threshold of stroke risk below which the bleeding risks of anticoagulant therapy do not result in a net clinical gain, and above which the benefits of stroke prevention outweigh the risks and consequences of major bleeding.
Although recent guidelines, from the American College of Chest Physicians (ACCP)(3) and the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS)(4), recommend that antithrombotic therapy be individualized to consider balance between risk of stroke and risk of major bleeding with treatment, bleeding risk is not explicitly considered in a quantitative manner. In short, the AHA/ACC/HRS guidelines, which now use the CHA2DS2VASc(5) to quantitate stroke risk, state it is reasonable to omit antithrombotic therapy for patients with a CHA2DS2VASc of 0, consider no antithrombotic therapy or treatment with an oral anticoagulant or aspirin for patients with a CHA2DS2VASc of 1, and recommend oral anticoagulants for patients with a CHA2DS2VASc ≥ 2. The guidelines suggest using the HAS-BLED(6) score to quantitate risk of major bleeding while receiving oral anticoagulant therapy (OAT – warfarin or DOACs), but do not make explicit recommendations about how to integrate this into decision-making.
We have previously described a computerized decision support tool that incorporates individual patient’s risk factor profiles for AF-related stroke and major bleeding.(7,8) Patient-level treatment recommendations are generated based upon projections for quality-adjusted life years (QALYs) calculated by a 29-state Markov decision analytic model. The previously described Atrial Fibrillation Decision Support Tool (AFDST) considered choices between OAT with warfarin, aspirin, or no antithrombotic therapy. We now describe an updated version of the AFDST that incorporates the DOACs dabigatran, apixaban, rivaroxaban, and edoxaban as additional strategies for thromboprophylaxis. The goal of this study was to examine the impact of DOACs on the tipping point in a real-world AF cohort in our University of Cincinnati (UC) Health system.
Methods
This study was undertaken in a single tertiary center. Our Institutional Review Board approved the study protocol.
Patient Population
5,121 unique adult patients (≥ 21 years of age) with non-valvular AF in the UC Health system identified using appropriate ICD-10 codes (I48.x) from the active problem lists for ambulatory visits and inpatient hospitalizations over the 1-year period (January through December 2016). Exclusion criteria include – Valvular heart disease (mitral valve disease (I05.x), aortic valve disease (I06.x), mitral and aortic valve disease (I08.x)), presence of prosthetic heart valve (Z95.2), or presence of xenogenic heart valve (Z95.3), or presence of other heart valve replacement (Z95.4).
AFDST
With the increasingly widespread adoption of electronic health records (EHRs), we have platforms at the point-of-care in which we can embed tools that “pull” data from the EHR and then “push” recommendations out as decision support. We have developed an AFDST that uses a decision analytic engine to generate patient-level recommendations for thromboprophylaxis.(7–10) Information required to calculate AF-related stroke risk using the CHA2DS2VASc(11), major hemorrhage using HAS-BLED(12), and intracerebral hemorrhage(12) rates are extracted from our EPIC® clinical data store (Clarity®) and are fed to the decision analytic engine. Stroke risk and bleeding risk (extracranial and intracerebral) are modified by appropriate measures of efficacy and relative hazards for each treatment based upon evidence in the published literature. Time in therapeutic range needed to calculate the HAS-BLED score is determined by interpolating international normalized ratio values over the past year, similar to the method by Rosendaal et al.(13) We retrieved current antithrombotic therapy from the active medication list.
The current version of the AFDST is an external web application that clinicians access by clicking on an activity button in the patient’s EHR. An AF data-mart consisting of a set of relevant Clarity® tables is refreshed every 24 hours. All patients in our system with a diagnosis of non-valvular AF are in the data-mart. Treatment recommendations are generated based upon projections for QALYs calculated by the decision analytic model.
The computational engine of the AFDST is a 29-state Markov state transition model examining strategies of - 1) no antihrombotic therapy, 2) aspirin, 3) warfarin, 4) dabigatran, 5) apixaban, 6) rivaroxaban, and 7) edoxaban (see Appendix Figure 1). The AFDST uses population-based utilities to value quality-of-life for health states in the model. We developed the model using a standard computer program (Decision Maker, Boston, MA) for model construction and analysis.
Efficacy of treatment and relative risk of complications including major bleeding and intracerebral hemorrhage were informed by literature review including meta-analyses(14,15) and network meta-analyses(16–18) of DOACs in general populations and in the elderly(19,20) along with systematic reviews, given the absence of head-to-head trials comparing DOACs to one another.(16,17) Logic was included to avoid recommending DOACs in patients with advanced chronic kidney disease. None of the DOACs were considered alternative treatments to warfarin or aspirin if the eGRF was < 30 ml/min/1.73m2. Edoxaban was not considered if estimated glomerular filtration rate (eGFR) was > 95 ml/min/1.73m2.
In order to evaluate the impact of DOACs, we analyzed model recommendations for all 5,121 members of our UC Health AF cohort, using structured query language (SQL) to generate a batch file containing all necessary values for clinical and demographic parameters. We then used Decision Maker’s remote control function to run a script file containing patient-level information through the decision analytic model. Results were stored to a text file that was loaded into a SQL database. The AFDST recommends the strategy resulting in the largest expected utility in quality-adjusted life-years (QALYs). We used 0.1 QALYs as a minimal clinically significant gain to consider one strategy better than another.
Review of Data
Risk of Ischemic Stroke
We used the CHA2DS2VASc score to calculate the risk of AF-related stroke without treatment (see Appendix Table 1).(5) While there are several mappings of stroke risk for any given CHA2DS2VASc score, we used rates from the SPORTIF III and V trials to be consistent with the rates quoted in the AHA/ACC/HRS guidelines.(21–23).
Efficacy of Thromboprophylactic Treatment
In each strategy, the risk of AF-related stroke was modified by the efficacy of treatment. We used a declining efficacy for aspirin with increasing age, such that efficacy is zero for patients ≥77 years of age, based on an analysis of the AF Investigators database of 8,932 patients and 17,685 years of observation.(24) We used an efficacy of 0.68 for warfarin (25), and adjusted for the efficacy of the DOACs by multiplying risk ratios determined in a network meta-analysis(18) times the monthly probability of AF-related stroke while taking warfarin (see Table 1). If the risk ratios were not statistically significant, we used a risk ratio of 1.0. We explored this assumption in sensitivity analyses (see results).
Table 1.
Data Required in the Analysis: Probabilities, Rates, and Quality-of-life.
| Parameter | Value | |||
|---|---|---|---|---|
| Annual Rate of Ischemic Stroke (untreated) | Based upon CHA2DS2VASc score (21) | |||
|
| ||||
| Efficacy of treatment | ||||
| with warfarin – | 0.68 (25) | |||
| aspirin – at age 50 | 0.30 (38) | |||
| at age 77 | 0.00 (38) | |||
|
| ||||
| Relative Risk of Ischemic Stroke (DOACs vs warfarin) | ||||
| dabigatran (150 mg bid) – | 0.76 (18) | |||
| apixaban (5 mg bid) – | 1.0 (18) | |||
| rivaroxaban (20 mg qd) – | 1.0 (18) | |||
| edoxaban (60 mg qd) – | 1.0 (18) | |||
|
| ||||
| Probable outcome of Ischemic Stroke | ||||
| Death – | 0.16 (39) | |||
| Permanent sequelae: | 0.44 (25,40) | |||
| with severe disability – | 0.69 (25,40) | |||
| with mild disability – | 0.31 (25,40) | |||
| Good recovery – | 0.40 (25,40) | |||
|
| ||||
| Annual Rate of major extracranial bleeding event | ||||
| (warfarin) – | Based upon HAS-BLED score (12) | |||
| (untreated) – | (HAS-BLED bleeding rate)/2.4 (26) | |||
| (aspirin) – | (Bleeding rate in untreated) * 1.08 (26) | |||
|
| ||||
| Relative Risk of major extracranial bleeding event (DOACs vs warfarin) | ||||
| dabigatran (150 mg bid) – | 0.697 (age < 75), 0.815 (age 75–79), 1.33 (age ≥ 80) (19) | |||
| apixaban (5 mg bid) – | 0.69 (18) | |||
| rivaroxaban (20 mg qd) – | 1.0 (18) | |||
| edoxaban (60 mg qd) – | 0.80 (18) | |||
|
| ||||
| Annual rate ICH‡ low risk referent group | ||||
| (untreated) | 0.0004 (12) | |||
|
| ||||
| Multivariate Hazard Ratios for ICH‡ | ||||
| (untreated)20 | (12) | |||
| Age < 65 | 1.0 | |||
| Age 65 – 74 | 1.97 | |||
| Age ≥ 75 | 2.43 | |||
| Female | 0.7 | |||
| Prior Ischemic Stroke | 1.21 | |||
| Hx of ICH | 8.92 | |||
| Hx of Severe Bleed | 3.1 | |||
| Hx of Myocardial Infarction | 0.82 | |||
| Hx of Ischemic Heart Disease | 0.81 | |||
| Hx of Poorly Controlled HTN† | 1.32 | |||
|
| ||||
| Annual rate Subdural Hematoma | ||||
| (untreated) | 0.00027 (25,28,41) | |||
|
| ||||
| Location of intracranial hemorrhage | ICH‡ | Subdural hematoma | ||
|
| ||||
| Relative hazard of bleeding (vs. no treatment) | ||||
| warfarin – | 4.1 (27,28) | 5.5 (28,42,43) | ||
| aspirin – | 1.84 (29–31) | 2.0 (44) | ||
|
| ||||
| Relative Risk of ICH‡ (DOACs vs warfarin) |
||||
| dabigatran (150 mg bid) – | 0.41 (18) | |||
| apixaban (5 mg bid) – | 0.42 (18) | |||
| rivaroxaban (20 mg qd) – | 0.67 (18) | |||
| edoxaban (60 mg qd) – | 0.47 (18) | |||
|
| ||||
| Lobar ICH | Deep ICH | Subdural hematoma | Extra cranial | |
|
| ||||
| Probable outcome from bleed (without warfarin/with warfarin)* | (45) | (45) | ||
| Death – | 0.19/0.38 | 0.21/0.41 | 0.267(31,46) | 0.024/0.05 |
| Severe long-term disability – | 0.43/0.43 | 0.44/0.42 | 0.07/0.09 (47) | 1 (31,46) |
| Mild long-term disability – | 0.20/0.11 | 0.19/0.10 | 0.40/0.50 (47) | |
| Good recovery – | 0.19/0.08 | 0.17/0.07 | 0.263/0.143 | |
|
| ||||
| Long-term symptoms | Base-Case Value of Quality-of-life | |||
|
| ||||
| Well | 1.0 | |||
|
| ||||
| Well while receiving anticoagulant therapy | 0.99 (48) | |||
|
| ||||
| Severe long-term disability | 0.11 (48) | |||
|
| ||||
| Mild long-term disability | 0.76 (48) | |||
|
| ||||
| Death | 0.0 | |||
|
| ||||
| Short-term symptoms | ||||
|
| ||||
| ICH | 0.79 | |||
|
| ||||
| Ischemic stroke | 0.79 | |||
|
| ||||
| Extracranial bleedξ | 0.84 | |||
|
| ||||
| Base-Case Value of Age-Adjusted Annual Excess Mortality | ||||
|
| ||||
| Stroke with long-term disability | 0.08 (49) | |||
|
| ||||
| Excess Mortality Risk for Comorbid Diseases (relative hazards or annual excess mortality rates)¶ | ||||
|
| ||||
| Type II Diabetes (relative hazards)¥ | ||||
| Age < 55 | 1.35 (50) | |||
| Age 55–64 | 0.79 (50) | |||
| Age 65–74 | 0.46 (50) | |||
| Age ≥75 | 0.19 (50) | |||
|
| ||||
| Chronic Kidney Disease (relative hazards)¥ | ||||
| Stage 3a (eGFR 45–59 ml/min/1.73m2) | 0.2 (51) | |||
| Stage 3b (eGFR 30–44 ml/min/1.73m2) | 0.8 (51) | |||
| Stage 4 (eGFR 15–29 ml/min/1.73m2) | 2.2 (51) | |||
| Stage 5 (eGFR < 15 ml/min/1.73m2) | 4.9 (51) | |||
|
| ||||
| CVA (annual excess mortality)£ | ||||
| Men Age < 70 | 0.0496 (52) | |||
| Men Age ≥ 70 | 0 1544 (52) | |||
| Women Age < 70 | 0.0336 (52) | |||
| Women Age ≥ 70 | 0.0983 (52) | |||
|
| ||||
| Congestive Heart Failure (annual excess mortality) | ||||
| Men | 0.21 (53) | |||
| Women | 0.17 (53) | |||
|
| ||||
| Hypertension (annual excess mortality) | 0.0011 (54) | |||
Poorly controlled hypertension – systolic BP ≥ 160 mmHg.
ICH – intracerebral hemorrhage
Assume outcomes of bleeding events for aspirin-treated patients are the same as for untreated patients.
Duration of short-term utility loss for major extracranial bleed is 12 months.
Excess annual mortality rates added to age and gender-adjusted annual mortality rates (μASR).
Multiply relative hazard times age and gender-adjusted μASR. eGFR – estimated glomerular filtration rate.
CVA – cerebral vascular event including ischemic stroke or intracerebral hemorrhage.
Risk of Major Bleeding
We used the HAS-BLED score to calculate the risk of major extracranial bleeding while receiving warfarin (see Appendix Table 2). We calculated the annual rate of major extracranial bleeding without warfarin by dividing the HAS-BLED-calculated rate by 2.4, the relative hazard of major bleeding while taking warfarin (see Table 1).(26) The risk of major extracranial bleeding while receiving aspirin was 1.08 times the rate off treatment.(26) The monthly probability of major extracranial bleeding while receiving treatment with DOACs was calculated by multiplying a risk ratio for each agent times the monthly probability of bleeding while receiving warfarin.(18) In the case of dabigatran, we used evidence from a meta-analysis describing an increasing hazard of major bleeding relative to warfarin, as age increased.
Risk of Intracerebral Bleeding
We independently modeled the risk of intracerebral hemorrhage (ICH) in untreated patients based on a multivariate regression model developed on a Swedish registry population of 90,490 untreated patients with AF.(12) We calculated the annual rate of ICH while receiving warfarin by multiplying this base rate times 4.1 (27,28), while that of aspirin-treated patients was 1.84 times that of untreated patients (see Table 1).(29–31) Annual rates of ICH while receiving treatment with DOACs were calculated by multiplying the annual ICH rate of warfarin-treated patients times a relative risk for each DOAC.(18)
Annual Mortality from Non-Explicitly Modeled Causes
Annual mortality from non-explicitly modeled causes was based on the most recent life-tables available from the Centers for Disease Control (2011 data, obtained in 2016) specific to age and gender.(32) In addition, patients faced an excess mortality risk for major comorbidities known from our collection of CHA2DS2VASc and HAS-BLED risk factors. These included type II diabetes, hypertension, congestive heart failure, chronic kidney disease, and prior cerebral vascular accident (see Table 1).
Role of the Funding Source
The study was funded by the Heart Rhythm Society through a grant from Boehinger-Ingelheim Pharmaceuticals, Inc. (BIPI) to Dr. Eckman. It was also supported by the National Center for Advancing Translational Sciences (NIH – UL1TR000077-05). The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The authors are solely responsible for the design and conduct of this study, all study analyses and drafting and editing of the paper.
Results
Patient characteristics are summarized in Table 2. Our AF cohort has significant comorbid diseases as reflected by mean CHA2DS2VASc and HAS-BLED scores of 3.5 and 2.4, respectively. A sample AFDST report is shown in Figure 1. For many patients the results do not favor a clear best treatment among DOACs, as they have similar efficacies and risks. From a decision analytic perspective, they are all reasonable choices. Therefore, the best choice for these patients must also include factors that are more subjective. We based these on our previous work assessing factors beyond treatment efficacy and life-threatening side effects that patients feel are important in decision-making. These include out-of-pocket cost, frequency of administration, reversal agents, need to take with food, significant non-life threatening side effects (such as dyspepsia), complexity of food-drug or drug-drug interactions, and need to have blood work done on a recurring basis (e.g., once or twice-monthly visits for INR). Appendix Figure 2 shows medication cards we developed to compare and contrast these more subjective issues (see Appendix Figures 3–4 for full patient-specific report).
Table 2.
Patient Characteristics (N= 5,121)
| Age, mean ± SD | 70.1 ± 13.5 |
| Female, n (%) | 2,197 (43) |
| White or Caucasian | 4173 (82) |
| Black or African American | 813 (16) |
| Asian | 39 (0.8) |
| Hispanic | 22 (0.4) |
| CHA2DS2VASc, mean ± SD | 3.5 ± 1.9 |
| HAS-BLED, mean ± SD | 2.4 ± 1.4 |
| Receiving oral anticoagulant therapy, n (%)† | 2,880 (56) |
| Receiving aspirin, n (%) | 1,399 (27) |
| Hypertension, n (%) | 3,226 (63) |
| Poorly Controlled Hypertension, n (%)£ | 1,080 (21) |
| Congestive Heart Failure, n (%) | 1,324 (26) |
| Type II Diabetes Mellitus, n (%) | 1,340 (26) |
| History of Stroke, n (%)¥ | 1,084 (21) |
| History of Intracranial Hemorrhage, n (%) | 174 (3) |
| History of Myocardial Infarction, n (%) | 746 (15) |
| History of Coronary Artery Disease, n (%) | 1,834 (36) |
| Vascular Disease, n (%)€ | 2,031 (40) |
| Abnormal Renal Function, n (%)₡ | 666 (13) |
| Abnormal Liver Function, n (%)∫ | 490 (10) |
| eGFR < 30 | 356 (7)₣ |
| eGFR < 15 | 136 (3)₣ |
| History of Bleeding╤ | 993 (19) |
| Labile INR, n (%)Ᵽ | 1,374 (27) |
| Non-aspirin NSAIDs, n (%) | 631 (12) |
| Significant Alcohol Use/Abuse, n (%) | 65 (1) |
| Current treatment discordant from AFDST- recommended treatment (n, %) | 1,812 (35)‡ |
| Current Treatment | n, (%) |
| No antithrombotic therapy | 844 (17) |
| Aspirin only | 1,275 (25) |
| Warfarin | 1,662 (32) |
| Aspirin/Clopidogrel | 120 (2) |
| Dabigatran | 118 (2) |
| Rivaroxaban | 591 (12) |
| Apixaban | 507 (10) |
| Edoxaban | 4 (0.08) |
Warfarin, dabigatran, rivaroxaban, apixaban, or edoxaban.
Poorly controlled hypertension – systolic blood pressure ≥ 160 mmHg.
History of Stroke – ischemic stroke, TIA, or thromboembolism.
Vascular Disease – prior myocardial infarction, peripheral arterial disease or aortic plaque.
Abnormal Renal Function – chronic dialysis, renal transplantation, or serum creatinine ≥ 2.26 mg/dl during past year.
Abnormal Liver Function – chronic hepatic disease (e.g., cirrhosis) or biochemical evidence of significant hepatic derangement (e.g., serum bilirubin ≥ 2× upper limit of normal, in association with elevations of AST, ALT, or ALP > 3× upper limit of normal, etc.)
Proportion of 4,802 patients with estimated glomerular filtration rate (eGFR) data available.
History of Bleeding – previous bleeding history or predisposition to bleeding (e.g., bleeding diathesis, anemia, etc.)
Labile INR – poor time in therapeutic range (< 60%).
AFDST – Atrial Fibrillation Decision Support Tool. Proportion is out of denominator of 4,573 patients for whom AFDST made a recommendation based on a gain of > 0.1 QALYs. No strong recommendation made for 549 patients (QALYs ≤ 0.1).
Figure 1.
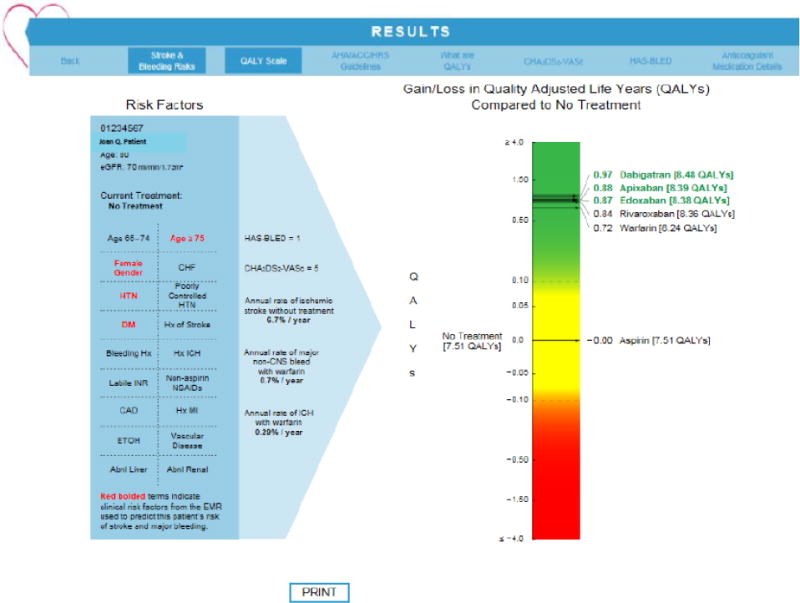
Sample Report from AFDST. Screen shot of report that appears in Epic Hyperspace frame when AFDSM is launched from a patient’s chart. Red, bolded items indicate clinical risk factors extracted from the AF data-mart used to predict the patient’s risk of stroke, major bleeding, intracranial bleeding and QALYs for each of the considered treatments. In this example, the patient is an 80-year old woman with a history of hypertension and type II diabetes. Her most recent estimated glomerular filtration rate (eGFR) is 70 mm/min/1.73m2. Her HAS-BLED is one and her CHA2DS2VASc score is five. Her annual rate of ischemic stroke without thromboprophylaxis is 6.7%. Her annual rate of major non-CNS bleeding while taking warfarin is 0.7%. This is an upper limit on risk of major bleeding, as the relative hazard of major bleeding is less than one for several of the DOACs. A separate model predicts the annual rate of intracerebral hemorrhage (ICH) while taking warfarin, 0.29% for this patient. This also is an upper limit, as the relative hazard of ICH is less than one for all of the DOACs. The graphic to the far right indicates gain or loss in QALYs for each of the considered strategies compared with no treatment. The visual analog scale is divided into three regions – green, indicating a clinically significant gain; red, indicating a clinically significant loss; and yellow, indicating a gain or loss less than 0.1 QALYs, which makes treatment too close to call as a recommended strategy compared with no treatment. For this patient, aspirin provides no benefit, while warfarin and the four DOACs all fall in the green range, providing net gains of 0.72 to 0.97 QALYs compared with no treatment. In particular, dabigatran, apixaban, and edoxaban all fall within 0.1 QALYs of each other, making them indistinguishable from a decision analytic perspective. In this example, all of the oral anticoagulants are reasonable choices. The patient’s decision between these agents needs to be guided by other more nuanced factors such as out-of-pocket cost, availability of reversal agent, number of doses per day, need for routine laboratory testing, etc. The clinician can click on the tab labelled “Print” to give the patient a copy of the report to take home. To facilitate this discussion in a typical shared decision-making encounter, the clinician would next click on the tab at the far right of the top ribbon, labeled “Anticoagulant Medication Details.” A graphic of medication cards (see appendix figure 2) detailing these factors that are important for patient choices between the various recommended oral anticoagulants appears, continuing to support the shared decision-making discussion.
Across the cohort of 5,121 AF patients, the AFDST recommended OAT (warfarin or one of the DOACs) for 4,134 (81%) patients, and no antithrombotic therapy or aspirin for 489 (9%). For 498 patients (10%) the gain afforded by any form of thromboprophylaxis (compared with no treatment) was less than 0.1 QALYs, thus no firm recommendation was given for these patients (i.e., decision was a “toss-up” between the best OAT and no antithrombotic therapy). We next compared AFDST recommendations to current treatment. As shown in panel A of Figure 2, along the descending diagonal, current treatment is concordant with recommendations for 2,474 patients (60%) currently receiving OAT and for 345 (70%) patients receiving either aspirin (149) or no thromboprophylaxis (196). The AFDST recommended OAT for 1,660 patients (40%) who currently are not receiving such treatment. Anticoagulation therapy for these patients would result in an estimated aggregate gain of 1,395 QALYs. No thromboprophylaxis (129) or aspirin (15) was recommended for 144 patients (30%) who are currently receiving OAT, resulting in an estimated loss of 113 QALYs due to potentially unnecessary thromboprophylaxis. In total, 1,508 QALYs could be gained were treatment changed to that recommended by the AFDST.
Figure 2.
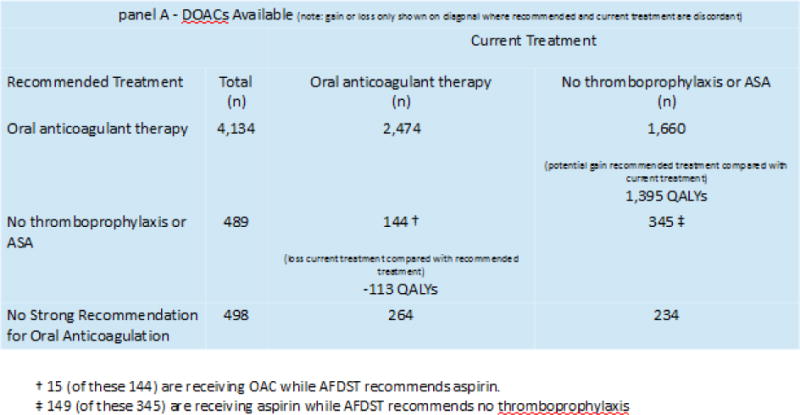
AFDST-Recommended-treatment compared with current treatment.
Panel A – results of AF cohort analysis when DOACs are available options for oral anticoagulant therapy. Recommendations and current treatment are concordant along the diagonal from the top left to the bottom right of the figure. In the discordant cells (bottom left to top right), the lower number represents the gain or loss in QALYs between recommended and current treatment. For instance, (bottom left), 119 QALYs could be gained were the 123 patients currently receiving treatment with oral anticoagulant therapy for whom the AFDST recommended either no thromboprophylaxis or aspirin, taken off oral anticoagulants. In the cell at the top right, 1,362 QALYs could be gained if these 1,673 patients currently not receiving oral anticoagulant therapy, were started on such treatment. In the setting of DOAC availability, an aggregate gain of 1,481 QALYs could potentially be achieved were treatment changed to that recommended by the AFDST.
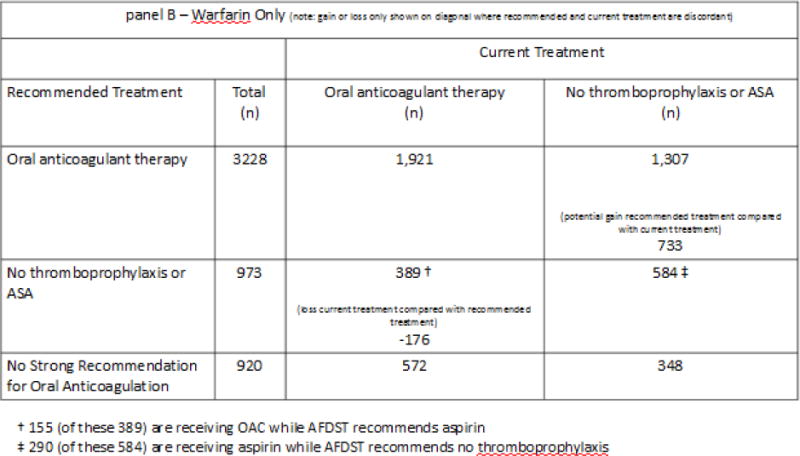
Panel B – results of AF cohort analysis when warfarin is the only available option for oral anticoagulant therapy. In this setting, an aggregate gain of 872 QALYs could potentially be achieved were treatment changed to that recommended by the AFDST.
In order to appreciate the impact of DOACs on the tipping point we also explored results assuming DOACs were not available and warfarin was the only option for oral anticoagulation. In this scenario, the AFDST recommended OAT for 3,228 (63%) patients, and no antithrombotic therapy or aspirin for 973 (19%) patients. For 920 patients (18%) the gain afforded by any form of thromboprophylaxis was less than 0.1 QALYs. We next compared AFDST recommendations to current treatment. As shown in panel B of Figure 2, along the descending diagonal, current treatment is concordant with recommendations for 1,921 patients currently receiving OAT and for 584 patients receiving either aspirin or no thromboprophylaxis. The AFDST recommended OAT for 1,307 patients who currently are not receiving such treatment. Anticoagulant therapy for these patients could result in an estimated aggregate gain of 733 QALYs. No thromboprophylaxis (234) or aspirin (155) was recommended for 389 patients who are currently receiving OAT, resulting in an estimated loss of 176 QALYs due to potentially unnecessary thromboprophylaxis. In total, 909 QALYs could potentially be gained were treatment changed to that recommended by the AFDST.
Of note, eGRF was < 30 ml/min/1.73m2 in 356 patients (7%) of the 4,802 for whom we had data on renal function. As a result, treatment with any of the DOACs was not considered as an option for these patients. We performed a sensitivity analysis in which we allowed use of DOACs for patients with eGFRs between 15 and 30 ml/min/1.73m2. We assumed efficacy and bleeding risk were the same despite renal dose adjustments. This had minimal impact on the overall proportion of patients recommended for anticoagulant therapy, 4,187 (82%), compared with 4,134 (81%) in the basecase analysis, or on the net gain in QALYs were treatment changed to that recommended by the AFDST (1,521 – basecase vs. 1,508 – sensitivity analysis).
In our basecase analysis, we used risk ratios of one for ischemic stroke and major bleeding risk, when confidence limits crossed one in the network meta-analysis. (18) We performed a sensitivity analysis on these assumptions, using relative risks of ischemic stroke of 0.96 and 0.94 for apixaban and rivaroxaban, respectively, compared with warfarin; and a relative risk of major bleeding of 1.03 for rivaroxaban compared with warfarin. Results of this sensitivity analysis showed little change in either the proportion of patients for whom anticoagulant therapy was recommended, 4,175 (82%), or on the net gain in QALYs were treatment changed to that recommended by the AFDST (1,517).
Finally, we wished to explore the magnitude of the gain or loss with OAT compared with no thromboprophylaxis across CHA2DS2VASc and HAS-BLED scores. Appendix Figure 5 shows the joint distribution of these two measures in our cohort. There is a clear correlation between these scores, highlighting the clinical challenge - as AF-related stroke risk increases, for many patients the risk of major bleeding while receiving OAT also increases. The majority of patients lie along a diagonal emanating from the upper left of the figure. Figure 3 shows the aggregate population gain or loss in QALYs with oral anticoagulation compared with no thromboprophylaxis in each cell. Panel A describes the situation in which DOACs are available, while panel B describes the situation in which only warfarin is available. In both of these figures, the greatest population gain occurs along a diagonal emanating from the upper left. Patients with a CHA2DS2VASc score of zero do not benefit from oral anticoagulation therapy in either scenario. When DOACs are available, significant population gains (>100 QALYs) achieved with oral anticoagulation are concentrated in the region between HAS-BLED scores of zero and three and CHA2DS2VASc scores between one and five. When warfarin is the only oral anticoagulant available, significant population gains (>100 QALYs) achieved with anticoagulation are concentrated in a smaller region between HAS-BLED scores of zero and two and CHA2DS2VASc scores between one and four.
Figure 3.
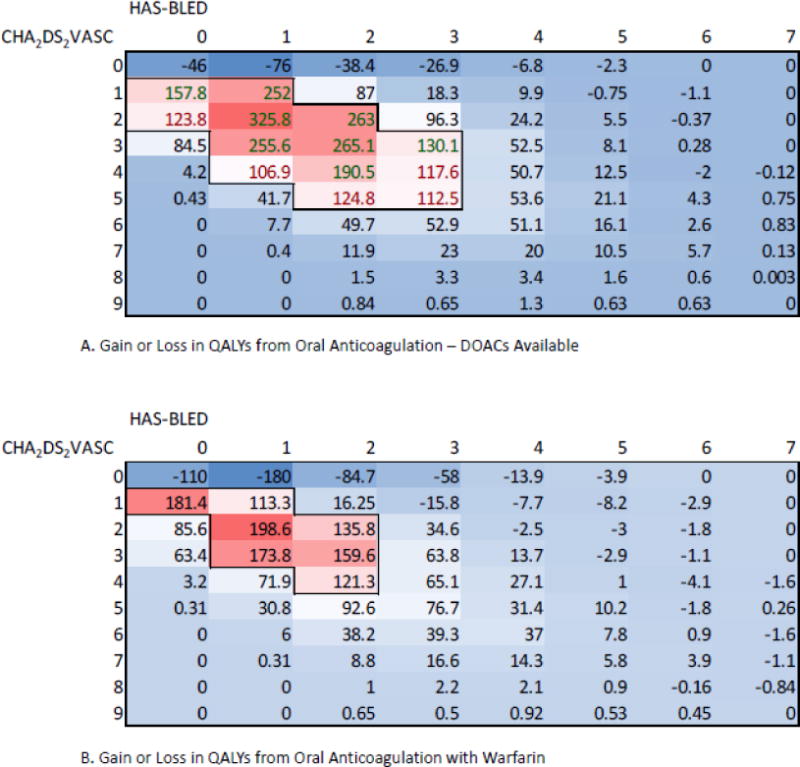
Heatmap showing gain or loss in QALYs for the UC Health AF cohort stratified by CHA2DS2VASc and HAS-BLED scores. More intense red colors correspond to regions of larger gain, while lighter blue colors correspond to regions of lesser gain. All gains or losses in both panels are relative to no thromboprophylaxis.
Panel A – results when DOACs are available options for anticoagulant therapy. Largest gains are within the region where CHA2DS2VASc scores are between one and five and HAS-BLED scores are between zero and three.
Panel B – results when warfarin is the only available option for oral anticoagulant therapy. Region of large population gains is smaller than in panel A, when DOACs are available. Largest gains are within the smaller region where CHA2DS2VASc scores are between one and four and HAS-BLED scores are between zero and two. In addition, the magnitude of the gains in even the most optimistic cells is smaller than in panel A.
Limitations
Our study had a number of important limitations. First, our model and analyses only considered single agent decisions for anticoagulation in patients with AF. We did not consider situations in which dual therapy (i.e., aspirin and an oral anticoagulant) or triple therapy (i.e., aspirin, clopidogrel, and an oral anticoagulant) are being used, as the clinical situations are generally even more complex, including scenarios of acute coronary syndrome and/or stenting. Second, we used a broad definition to exclude AF patients with valvular heart disease, as ICD-10 codes do not specify degree of stenosis or regurgitation. Thus, we excluded even patients with mild disease. Seven hundred and sixty-eight patients were excluded from our cohort for valvular heart disease or valve replacement.
Another important limitation was the use of the active problem list and the history list from the EHR as the source of diagnostic information used to inform the CHA2DS2VASc and HAS-BLED scores. It is possible that inaccurate information gets into the medical record at the level of provider data entry. To provide safeguards for this in clinical practice, the AFDST provides clinicians with the opportunity to review diagnostic information extracted from the EHR and either add or remove clinical risk factors. In a prior study of the AFDST, we found that clinicians made such changes in a total of roughly one third of patients.(7) However, the most common change was the addition of coronary artery disease as a diagnosis not captured on our query of the active problem lists. Although such changes may have affected the magnitude of the gain or loss with OAT, it was rare that they caused the AFDST recommendation to change.
Renal dosing adjustments, based on how creatinine clearance (CrCl) was measured in clinical trials evaluating the DOACs, used CrCl calculated with the Cockcroft-Gault formula. However, as a practical matter, many electronic health record systems, including our Epic® installation, provide automated calculations of creatinine clearance using estimated GFR as calculated by the CKD-epi formula.(33) Studies have shown this calculation to be more accurate in assessing renal function than either the MDRD or the Cockcroft-Gault formula. As a result, the AFDST uses the eGFR as calculated by the CKD-epi formula as a proxy for CrCl. Third, in calculating both efficacy of the DOACs in preventing ischemic stroke, and major bleeding, when relative risks were not statistically significant (i.e., confidence limits of RRs crossed one), we assumed these risks were not statistically different from warfarin. We performed sensitivity analyses to explore the impact of this assumption on our results and it was quite small. Finally, the decision support tool we are using in our health system has been programmed to be very conservative concerning DOAC use in patients with advanced CKD. Numerous studies have documented a growing national problem with inappropriate dosing of DOACs, particularly overdosing in patients with CKD.(34,35) This problem is compounded by the dynamic and progressive nature of CKD, and without careful monitoring of renal function and appropriate dose adjustments, patients can be exposed to excessive risks of major bleeding. Therefore, the AFDST does not recommend DOACs as an option for oral anticoagulation when the eGFR is less than 30 ml/min/1.73m2, even though manufacturer dosing guidelines allow for renal dosing adjustments for eGFR as low as 15 ml/min/1.73m2. This is consistent with current guidelines that still suggest the avoidance of DOACs in patients with CKD stages 4–5.(3,4) We performed sensitivity analyses to explore the impact of this assumption on results. Once more, the impact was quite small, leaving the results of our basecase analysis robust to these assumptions.
Discussion
Our analysis suggests that the tipping point has indeed changed given the introduction of a new generation of oral anticoagulants. In our study, projecting the potential aggregate net gain in QALYs in our health system’s AF population, the availability of these agents, with an improved safety profile relative to major bleeding events, particularly and at least equivalent efficacy in the prevention of ischemic strokes, increased the proportion of patients for whom oral anticoagulation therapy was recommended from 63% were warfarin the only option to 81% when DOACs are included. The availability of DOACs also increased the projected gain in QALYs, achieved by recommended treatment, by more than 1,500, vs 909 QALYs were warfarin the only oral anticoagulant available. In addition, the range of CHA2DS2VASc and HAS-BLED score combinations (Figure 3) for which oral anticoagulation therapy is favored increased when DOACs were included as options for anticoagulant therapy. When examining the aggregate population gains or losses in QALYs shown in Figure 3, it is important to note that the CHA2DS2VASc and HAS-BLED scores are not the only factors that are changing. Many other clinical parameters that affect life expectancy and quality-of-life are changing simultaneously, depending upon their joint distribution with risk factors in the CHA2DS2VASc and HAS-BLED algorithms. In particular, these include the annual rate of ICH for which the AFDST uses a separate prediction model, and competing forces of mortality due to chronic diseases such as diabetes, hypertension, heart failure, and chronic kidney disease. Finally, with the availability of DOACs, the proportion of patients for whom aspirin is optimal has become exceedingly small. When warfarin was the only available oral anticoagulant, the AFDST recommended aspirin for 6% of patients, while aspirin was recommended for only 0.5% of patients when DOACs were available.
An important issue we did not explore in this study is the possible declining risk of ischemic stroke among AF patients.(2) The AFDST uses stroke rates referenced by the AHA/ACC/HRS guidelines for the CHA2DS2VASc.(21–23) Reported stroke rates in other AF cohorts are either higher or lower for any given CHA2DS2VASc score.(36) In particular, stroke rates in ATRIA, the community-based Kaiser Permanente AF cohort are lower for CHA2DS2VASc scores less than eight.(37) It is unclear whether this variation is due to differences in methodology used to assess strokes, or true differences in stroke rates across populations. The consequence of lower stroke rates would be to move the tipping point in the opposite direction, decreasing the proportion of AF patients for whom OAT results in a net benefit. Ultimately, the balance of these two forces – the availability of oral anticoagulants with an improved safety profile, and a possible declining risk of AF-related stroke, will determine the true tipping point. This has great importance for both guideline recommendations and decision support tools, such as the AFDST. What do we do in the meantime? We cannot afford to abrogate decision-making for these patients and must use the best tools available. However, we must remain mindful of the variation and uncertainty that surrounds reported stroke rates and use guidelines and decision support tools as aids and not replacements for thoughtful decision-making.
Supplementary Material
Acknowledgments
Dr. Flaherty serves as a consultant to Janssen and serves on an advisory board for CSL Behring and Portola. He also serves on a speaker’s bureau for CSL Behring and Janssen. Dr. Eckman also has current or recent investigator-initiated grant funding from Pfizer Educational Group, Bristol-Myers Squibb/Pfizer Education Consortium, and the Cystic Fibrosis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The other authors report no conflicts.
References
- 1.Casciano JP, Dotiwala ZJ, Martin BC, Kwong WJ. The costs of warfarin underuse and nonadherence in patients with atrial fibrillation: a commercial insurer perspective. J Manag Care Pharm. 2013;19:302–316. doi: 10.18553/jmcp.2013.19.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011;4:14–21. doi: 10.1161/CIRCOUTCOMES.110.958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S–575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014 doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 6.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2011;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 7.Eckman MH, Lip GY, Wise RE, et al. Impact of an Atrial Fibrillation Decision Support Tool on thromboprophylaxis for atrial fibrillation. Am Heart J. 2016;176:17–27. doi: 10.1016/j.ahj.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Eckman MH, Wise RE, Speer B, et al. Integrating real-time clinical information to provide estimates of net clinical benefit of antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2014;7:680–686. doi: 10.1161/CIRCOUTCOMES.114.001163. [DOI] [PubMed] [Google Scholar]
- 9.Eckman MH, Lip GY, Wise RE, et al. Using an Atrial Fibrillation Decision Support Tool for Thromboprophylaxis in Atrial Fibrillation: Effect of Sex and Age. J Am Geriatr Soc. 2016;64:1054–1060. doi: 10.1111/jgs.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckman MH, Wise RE, Naylor K, et al. Developing an Atrial Fibrillation Guideline Support Tool (AFGuST) for shared decision making. Curr Med Res Opin. 2015;31:603–614. doi: 10.1185/03007995.2015.1019608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 13.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 14.Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. The American journal of cardiology. 2012;110:453–460. doi: 10.1016/j.amjcard.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 16.Adam SS, McDuffie JR, Ortel TL, Williams JW., Jr Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med. 2012;157:796–807. doi: 10.7326/0003-4819-157-10-201211200-00532. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Outes A, Terleira-Fernandez AI, Calvo-Rojas G, Suarez-Gea ML, Vargas-Castrillon E. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups. Thrombosis. 2013;2013:640723. doi: 10.1155/2013/640723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tawfik A, Bielecki JM, Krahn M, et al. Systematic review and network meta-analysis of stroke prevention treatments in patients with atrial fibrillation. Clin Pharmacol. 2016;8:93–107. doi: 10.2147/CPAA.S105165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barco S, Cheung YW, Eikelboom JW, Coppens M. New oral anticoagulants in elderly patients. Best Pract Res Clin Haematol. 2013;26:215–224. doi: 10.1016/j.beha.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62:857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 21.Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731–2738. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 22.Olsson SB, Executive Steering Committee of the SIIII Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691–1698. doi: 10.1016/s0140-6736(03)14841-6. [DOI] [PubMed] [Google Scholar]
- 23.Poli D, Antonucci E, Grifoni E, Abbate R, Gensini GF, Prisco D. Stroke risk in atrial fibrillation patients on warfarin. Predictive ability of risk stratification schemes for primary and secondary prevention. Thromb Haemost. 2009;101:367–372. [PubMed] [Google Scholar]
- 24.van Walraven C, Hart RG, Connolly S, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the atrial fibrillation investigators. Stroke. 2009;40:1410–1416. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 25.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 26.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 27.Fang MC, Go AS, Hylek EM, et al. Age and the risk of warfarin-associated hemorrhage: the anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006;54:1231–1236. doi: 10.1111/j.1532-5415.2006.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36:1801–1807. doi: 10.1161/01.STR.0000174189.81153.85. [DOI] [PubMed] [Google Scholar]
- 30.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: a meta-analysis of randomized controlled trials. JAMA. 1998;280:1930–1935. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- 31.van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–2448. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 32.Arias E. United States Life Tables, 2011. 2015;64(11) https://www.cdc.gov/nchs/products/life_tables.htm. Accessed 4/30/82016. [PubMed] [Google Scholar]
- 33.Flamant M, Haymann JP, Vidal-Petiot E, et al. GFR estimation using the Cockcroft-Gault, MDRD study, and CKD-EPI equations in the elderly. Am J Kidney Dis. 2012;60:847–849. doi: 10.1053/j.ajkd.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Steinberg BA, Shrader P, Thomas L, et al. Off-Label Dosing of Non-Vitamin K Antagonist Oral Anticoagulants and Adverse Outcomes: The ORBIT-AF II Registry. J Am Coll Cardiol. 2016;68:2597–2604. doi: 10.1016/j.jacc.2016.09.966. [DOI] [PubMed] [Google Scholar]
- 35.Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-Vitamin K Antagonist Oral Anticoagulant Dosing in Patients With Atrial Fibrillation and Renal Dysfunction. J Am Coll Cardiol. 2017;69:2779–2790. doi: 10.1016/j.jacc.2017.03.600. [DOI] [PubMed] [Google Scholar]
- 36.Quinn GR, Severdija ON, Chang Y, Singer DE. Wide Variation in Reported Rates of Stroke Across Cohorts of Patients With Atrial Fibrillation. Circulation. 2017;135:208–219. doi: 10.1161/CIRCULATIONAHA.116.024057. [DOI] [PubMed] [Google Scholar]
- 37.Fang MC, Go AS, Chang Y, Borowsky L, Pomernacki NK, Singer DE. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2008;51:810–815. doi: 10.1016/j.jacc.2007.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Walraven C, Hart RG, Connolly S, et al. Effect of Age on Stroke Prevention Therapy in Patients With Atrial Fibrillation. The Atrial Fibrillation Investigators. Stroke. 2009 doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 39.Sherman DG, Kim SG, Boop BS, et al. Occurrence and characteristics of stroke events in the Atrial Fibrillation Follow-up Investigation of Sinus Rhythm Management (AFFIRM) study. Arch Intern Med. 2005;165:1185–1191. doi: 10.1001/archinte.165.10.1185. [DOI] [PubMed] [Google Scholar]
- 40.Penado S, Cano M, Acha O, Hernandez JL, Riancho JA. Stroke severity in patients with atrial fibrillation. Am J Med. 2002;112:572–574. doi: 10.1016/s0002-9343(02)01063-x. [DOI] [PubMed] [Google Scholar]
- 41.Wintzen AR, Tijssen JG. Subdural hematoma and oral anticoagulant therapy. Arch Neurol. 1982;39:69–72. doi: 10.1001/archneur.1982.00510140003001. [DOI] [PubMed] [Google Scholar]
- 42.Wintzen AR, de Jonge H, Loeliger EA, Bots GT. The risk of intracerebral hemorrhage during oral anticoagulant treatment: a population study. Ann Neurol. 1984;16:553–558. doi: 10.1002/ana.410160505. [DOI] [PubMed] [Google Scholar]
- 43.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26:1471–1477. doi: 10.1161/01.str.26.8.1471. [DOI] [PubMed] [Google Scholar]
- 44.Bleeding during antithrombotic therapy in patients with atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. Arch Intern Med. 1996;156:409–416. [PubMed] [Google Scholar]
- 45.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 46.Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med. 2007;120:700–705. doi: 10.1016/j.amjmed.2006.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 48.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–1836. [PubMed] [Google Scholar]
- 49.Lai SM, Alter M, Friday G, Sobel E. Prognosis for survival after an initial stroke. Stroke. 1995;26:2011–2015. doi: 10.1161/01.str.26.11.2011. [DOI] [PubMed] [Google Scholar]
- 50.Tancredi M, Rosengren A, Svensson AM, et al. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 51.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 52.Bronnum-Hansen H, Davidsen M, Thorvaldsen P, Danish MSG. Long-term survival and causes of death after stroke. Stroke. 2001;32:2131–2136. doi: 10.1161/hs0901.094253. [DOI] [PubMed] [Google Scholar]
- 53.Roger VL. Epidemiology of heart failure. Circ Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Writing Group M. Lloyd-Jones D, Adams RJ, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


