Figure 1. Engineering and validation of CDK8as/as HCT116 cells.
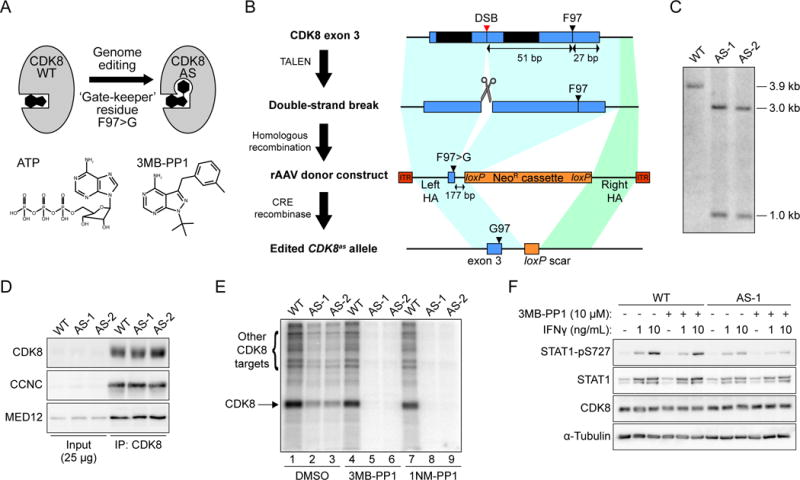
(A) Cartoon depicting the creation of ‘analog sensitive’ CDK8-AS by altering the ‘gate-keeper’ residue in the kinase active site. Structures of ATP and the analog 3MB-PP1 are shown for reference.
(B) Outline of genome editing strategy to generate CDK8as/as HCT116 cells. Each round entailed generation of a DNA double-strand break (DSB) in exon 3 of CDK8, using a transcription activator-like effector nuclease (TALEN) pair, followed by homologous recombination with a recombinant adeno-associated virus (rAAV)-based repair donor construct containing the F97G mutation and a loxP-flanked neomycin resistance (NeoR) cassette, selection for resistance, and finally removal of the NeoR cassette using transient expression of CRE recombinase. TAL-1 and TAL-2, TALEN binding sites; HA, homology arm; ITR, inverted terminal repeat.
(C) Southern blot hybridization analysis of AvrII-digested genomic DNA from WT and two independent homozygous CDK8as/as clones (AS-1 and AS-2), using a probe spanning the novel AvrII restriction site introduced along with the F97G mutation in CDK8 exon 3. Fragment sizes in kilo-base pairs are indicated at right.
(D) Western blot analysis of CDK8, Cyclin C (CCNC) and MED12 levels for inputs (2.5%) and CDK8 immunoprecipitations from WT and AS lysates.
(E) In vitro kinase assay with CDK8 immunoprecipitated material, as in D, showing labelling of proteins with 32P-ATP in the presence of vehicle (DMSO) or the ATP analogs 3MB-PP1 (10 μM) or 1NM-PP1 (10 μM). Arrows indicate bands representing phosphorylation of CDK8 itself, or additional proteins present in the immunoprecipitation.
(F) Western blot showing levels of S727-phosphorylated STAT1 (STAT1-pS727), total STAT1, and CDK8 in HCT116 WT or CDK8 AS-1 cell lysates following treatment with interferon gamma (IFNγ) and/or 10 μM 3MB-PP1.
