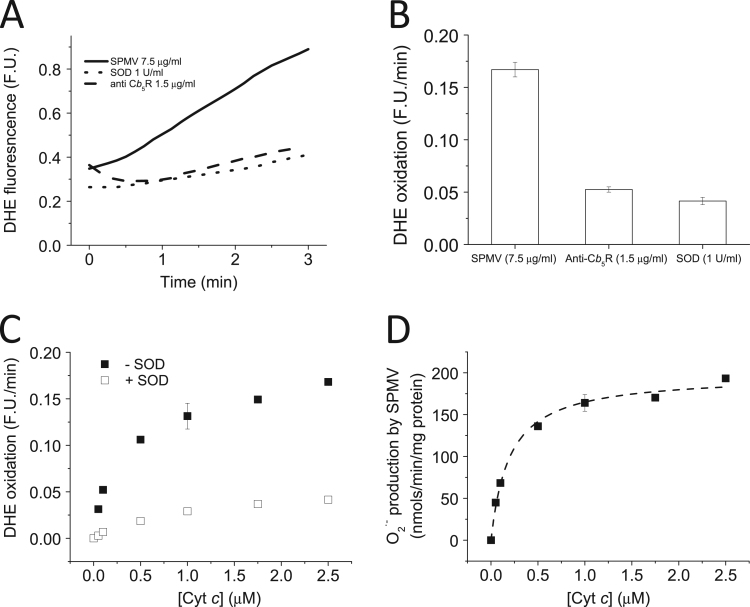
Fig. 1.
Cytcstimulated NADH-dependent O2·-production by SPMV. Panel A : Kinetics of the NADH dependent DHE oxidation by SPMV measured by fluorescence in the absence and presence of SOD (1 U/mL) and anti- Cb5R antibody (1.5 μg/mL). DHE oxidation was measured at 37 °C in potassium phosphate 20 mM plus DTPA 0.1 mM (pH 7.0), using a Perkin Elmer spectrofluorimeter with 470 nm and 605 nm excitation and emission wavelengths, respectively, and 10 nm excitation and emission slits. Representative traces of DHE oxidation by SPMV (7.5 μg/mL) in the presence of NADH (50 μM), oxidized Cyt c (Fe3+) (2.5 μM) and DHE (2 μM),in the presence of 1.5 μg/mL anti-Cb5R (dashed line) or 1 U/mL SOD (dotted line) are shown. Panel B: Quantification of the inhibition induced by anti-Cb5R (1.5 μg/mL) and SOD (1 U/mL) on the DHE oxidation rate by SPMV (7.5 μg/mL) in the presence of NADH (50 μM) and oxidized Cyt c (Fe3+) (2.5 μM). Panel C: Dependence of the NADH-dependent DHE oxidation rate by SPMV (7.5 μg/mL) upon Cyt c concentration in the absence (filled squares) or in the presence of SOD (1 U/mL) (open squares). Panel D: NADH dependent O2·- production by SPMV (7.5 μg/mL) dependence upon Cyt c concentration, measured with DHE. All the results shown in this Figure are the average (± standard errors) of experiments done by triplicate.