Fig. 3.
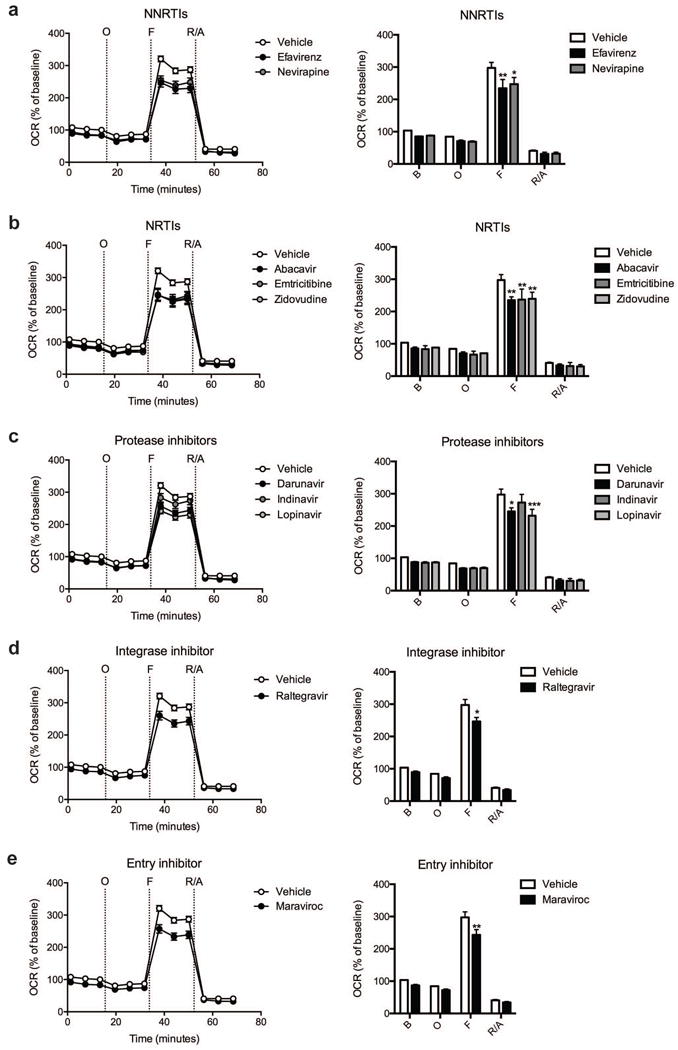
Effect of ARV drug treatment on striatal nerve terminal respiration. The OCR was measured in striatal nerve terminals exposed to vehicle or 25 μM ARV drug for 2 hours. Left: Graphical representation of the relative OCR responses over time expressed as a percent response from baseline (third measurement, before oligomycin injection); sequential additions are indicated as O (5 μM oligomycin), F (4 μM FCCP), and R/A (2 μM rotenone and 2 μM antimycin A). Right: Quantification of the mean OCR in striatal nerve terminals exposed to (a) NNRTIs (efavirenz or nevirapine), (b) NRTIs (abacavir, emtricitabine, or zidovudine), (c) protease inhibitors (darunavir, indinavir, or lopinavir), (d) integrase inhibitor (raltegravir), or (e) entry inhibitor (maraviroc) is shown for respiration under baseline conditions (B) and after the sequential additions (O, F, and R/A). Data (mean ± SEM; n = 3) were compared with those for vehicle control and the complete data set was analyzed by ANOVA with Bonferroni post-hoc test; significance versus control: *p < 0.05, **p < 0.01, ***p < 0.001. For ease of visualization the data is presented with the vehicle control compared to each class of ARV drugs individually.
