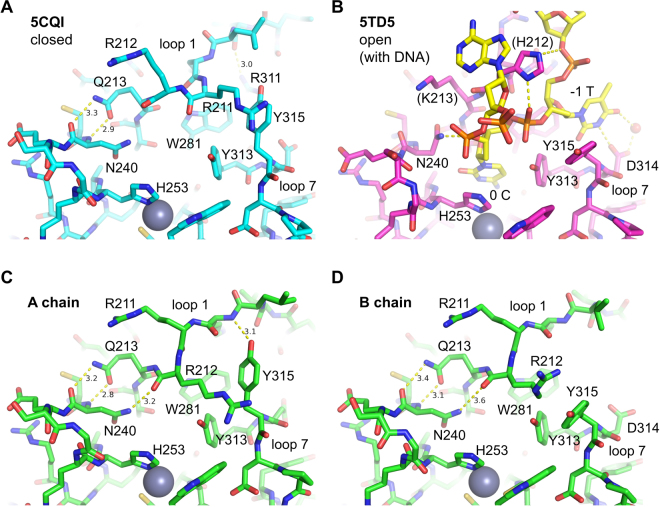
Figure 2.
Detailed interactions in the various active site conformations shown in Fig. 1. In all DNA-free structures of A3Bctd (A,C,D), Gln213 at the C-terminal end of loop 1 is hydrogen-bonded to the Asn240 main chain. This positioning of Gln213 is not compatible, for steric reasons, with the conformation of Asn240 observed in the ssDNA-bound A3Bctd (B). The δ2 nitrogen atom of Asn240 makes a DNA backbone interaction in the DNA complex. The Asn240 side chain is instead interacting with Arg212 backbone in the new apo crystal structures (C,D). Tyr315 is in the closed (gauche +) conformation in (A,C) whereas it is in the open (trans) conformation in (B,D). In (B), the loop 1 residues including His212 are APOBEC3A-derived. The yellow dotted lines denote hydrogen bonds.