Table 3.
Grignard scope
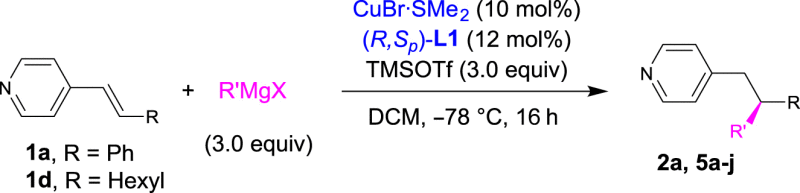
| |||||
|---|---|---|---|---|---|
| Entry | R′MgX | R | Product | Yield (%)a | ee (%)b |
| 1 | EtMgBr | Ph | 2a | 94 | 93 |
| 2 | PrMgCl | Ph | 5a | 75 | 93 |
| 3 | HexMgBr | Ph | 5b | 81 | 95 |
| 4 | i-PentMgBr | Ph | 5c | 65 | 97 |
| 5 | i-BuMgBr | Hexyl | 5d | 56 | 64 |
| 6c | c-PentMgBr | Hexyl | 5e | 54 | 89 |
| 7 | CH2=CH(CH2)2MgBr | Hexyl | 5f | 91 | 93 |
| 8 | CH2=CH(CH2)3MgBr | Ph | 5g | 66 | 90 |
| 9 | Ph(CH2)2MgBr | Hexyl | 5 h | 89 | 97 |
| 10d | MeMgBr | Hexyl | 5i | 50 | 93 |
| 11e | PhMgBr | Hexyl | 5j | 84 | 0 |
aReported yields are for isolated products
bDetermined by chiral HPLC
c4. equiv. of Grignard was used
dReaction was carried out at 0 °C for 5 h
eLigand L10 was used (see Supplementary Table 1 for L10 structure)
