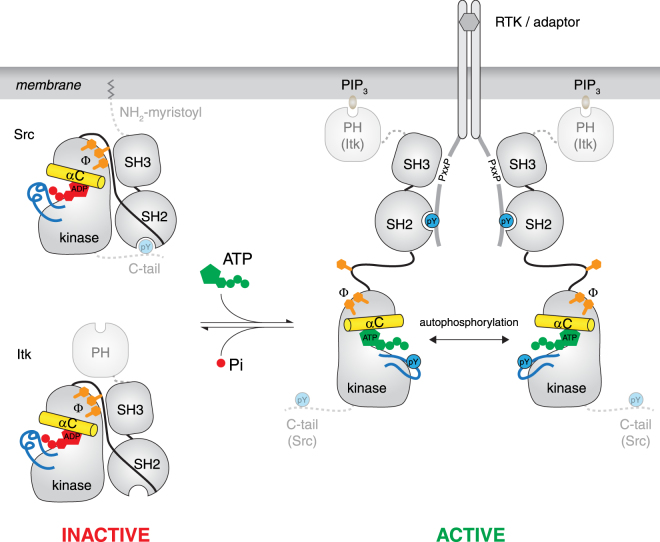
Figure 5.
Model for the allosteric activation of Src and Tec family kinases. Src and Tec kinases adopt a closed, autoinhibited conformation in the cytosol of unstimulated cells stabilized by ADP. Activation of receptor tyrosine kinases in the membrane leads to the phosphorylation of motifs in their cytoplasmic domains and the consequent recruitment of phosphotyrosine-binding SH2-domain containing proteins, including Src and Tec kinases. Engagement of SH2 and SH3 binding motifs in the receptor or adaptor proteins leads to activation of these kinases. Displacement of the SH3 domain from contacts with the N-terminal lobe of the kinase domain disrupts a hydrophobic stack that stabilizes the inactive state, leading to a conformational change that drives the exchange of ADP for ATP, trans-autoactivation, and downstream substrate phosphorylation. Additional regulatory adaptations restrict Itk activity to PIP3-containing membranes on account of its regulatory PH domain, while the C-terminal tail of Src prevents spurious activation at the membrane. (yellow, αC helix; orange, hydrophobic stack; blue, activation loop; red, ADP; green, ATP; Φ, hydrophobic amino acid).