Figure 2.
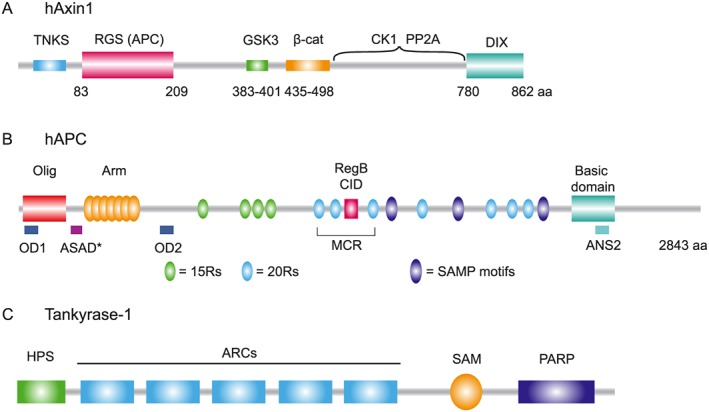
Structural organization of axin, APC and tankyrase (TNKS). (A) Human axin carries two structured domains, indicated as the N‐terminal RGS domain and C‐terminal DIX domain. The N‐terminal region contains a TNKS binding motif. The central intrinsically disordered region of axin contains binding motifs for GSK3, β‐catenin (β‐cat), CK1 and PP2A. (B) Human APC contains multiple domains including the oligomerization domain (Olig, red), Armadillo repeat domain (Arm, yellow), Region B (RegB, pink) or CID and the basic domain (aqua blue). The β‐catenin binding 15‐mer repeats (15Rs; green) and 20‐mer repeats (20Rs; blue) and axin binding SAMP motifs (purple) are indicated. Cancer mutations in APC frequently truncate the APC protein in the mutational cluster region (MCR). Self‐oligomerization of APC is facilitated by N‐terminal OD1 and OD2 and C‐terminal ANS2. Drosophila APC can self‐polymerize via the ASAD domain that shows sequence conservation in human APC, shown here as ASAD*. (C) Human TNKS1 contains five Ankyrin repeat clusters (ARCs; blue), a polymerization domain (SAM, yellow) and a C‐terminal catalytic PARP (purple) domain. The N‐terminus contains an HPS domain (green), a homopolymeric run of histidine, proline and serine of which the function is unknown.
