Abstract
H7 subtype influenza viruses have demonstrated an ocular tropism in humans, causing conjunctivitis and not respiratory symptoms in many infected individuals. However, the molecular determinants which confer ocular tropism are still poorly understood. Here, we used a murine model of ocular inoculation to demonstrate that H7 influenza viruses are more likely to cause infection following ocular exposure than are non-H7 subtype viruses. We included investigation regarding the potential role of several properties of influenza viruses with murine infectivity following ocular inoculation, including virus lineage, pathogenicity, and HA cleavage site composition. Furthermore, we examined the potential contribution of internal proteins to murine ocular infectivity. These studies establish a link between H7 subtype viruses and the risk of heightened infectivity in a mammalian species following ocular exposure, and support the development of non-traditional inoculation methods and models to best understand the human risk posed by influenza viruses of all subtypes.
Keywords: Influenza, Tropism, Mouse model, Conjunctivitis, Ocular
1. Introduction
H7 subtype influenza viruses, with the notable exception of H7N9 viruses, are typically associated with ocular complications in humans, with ~80% of reported cases presenting with conjunctivitis (Belser et al., 2009a). In contrast, the majority of non-H7 subtype avian and human influenza viruses generally present with respiratory symptoms in humans, with ocular complications only rarely reported (Belser et al., 2013c). However, the human eye and surrounding conjunctiva represent a secondary mucosal surface bearing permissive receptors susceptible to virus infection (Kumlin et al., 2008), underscoring that ocular exposure to influenza viruses represents a potential route of virus entry for multiple virus subtypes. Thus, elucidating how influenza viruses use the eye as a means to establish a respiratory infection, and determining the viral and host features which govern ocular tropism, will offer critical information which can guide public health responses during outbreaks of H7 subtype virus.
The capacity for influenza viruses to cause disease following ocular exposure has been examined in several in vitro and in vivo models. Multiple human ocular cell types (including corneal and conjunctival cells) have demonstrated the ability to support productive replication of both human and avian influenza viruses (Belser et al., 2011b; Chan et al., 2010; Michaelis et al., 2009). Similarly, ferrets (challenged with either a liquid or aerosol inoculum) have revealed a capacity for both human and avian influenza viruses to use the eye as a portal of entry to establish a productive respiratory infection (Aamir et al., 2009; Belser et al., 2014, 2012a). In contrast, ocular inoculation of mice has shown that H7 influenza viruses appear to more frequently infect following inoculation and are detected in the eye more often than other virus subtypes (Belser et al., 2009b; Sun et al., 2009; Tannock et al., 1985).
Identifying a mammalian model which recapitulates the tropism observed in humans offers an opportunity to investigate which features of H7 subtype viruses confer ocular tropism or ocular infectivity in vivo. Here, we assess numerous viral properties for their influence on murine infectivity following ocular inoculation, including the contribution of surface glycoprotein composition, HA cleavage site, lineage, receptor binding preference, and internal gene constellation. We find that, in agreement with human epidemiological reports, H7 subtype viruses are more likely to cause infection following ocular inoculation in a mammalian model, and are more likely than other virus subtypes to replicate within ocular tissue, including H5N1 viruses. Identification of properties which do or do not confer ocular tropism in a mammalian host provides needed information to more precisely determine the molecular correlates which govern this attribute in humans.
2. Methods
2.1. Viruses
Influenza A viruses included in the analyses in this study are shown in Tables 1 and 3, with ocular pathotyping data for each virus either published previously (as indicated by the presence of a reference in Table 1) or generated for this study. All viruses were propagated in the allantoic cavity of 10-day-old embryonating chicken eggs, with the exception of pandemic 1918 and 2009 H1N1 viruses, which were propagated in Madin-Darby canine kidney (MDCK) cells, as referenced in Table 1. The 50% egg infectious dose (EID50) titer for each virus stock was calculated by the method of Reed and Muench (Reed and Muench, 1938). The PFU titer was determined by standard plaque assay in MDCK cells (Zeng et al., 2007). All experiments with avian-origin viruses were conducted under biosafety level 3 containment, including enhancements required by the U.S. Department of Agriculture and the Division of Select Agents and Toxins/CDC (Chosewood and Wilson, 2009).
Table 1.
Influenza viruses tested for infectivity by the ocular route in mice.
| Subtype | IVPIa | Virus name | Isolate source | # inocb | # infc | References |
|---|---|---|---|---|---|---|
| H7N2 | LPAI | A/New York/107/2003 | Human respiratory case | 6 | 0 | (Belser et al., 2009b) |
| H7N2 | LPAI | A/chicken/Connecticut/260413-2/2003 | avian | 6 | 0 | this study |
| H7N3 | HPAI | A/Canada/504/2004 | Human conjunctivitis case | 21 | 16 | (Belser et al., 2012b, 2009b) |
| H7N3 | LPAI | A/Canada/444/2004 | Human conjunctivitis case | 12 | 10 | (Belser et al., 2009b) |
| H7N3 | LPAI | A/Red Knot/New Jersey/1523470/2006 | avian | 6 | 1 | (Belser et al., 2013a) |
| H7N3 | HPAI | A/Mexico/InDRE7218/2012 | Human conjunctivitis case | 16 | 15 | (Belser et al., 2013a) |
| H7N7 | HPAI | A/Netherlands/219/2003 | Human fatal case | 20 | 20 | (Belser et al., 2012b, 2009b) |
| H7N7 | HPAI | A/Netherlands/230/2003 | Human conjunctivitis case | 12 | 8 | (Belser et al., 2009b) |
| H7N7 | HPAI | A/chicken/Netherlands/1/2003 | avian | 6 | 2 | (Belser et al., 2009b) |
| H7N8 | HPAI | A/turkey/Indiana/1403/2016 | avian | 9 | 1 | (Sun et al., 2016a) |
| H7N8 | LPAI | A/turkey/Indiana/1573-2/2016 | avian | 10 | 0 | (Sun et al., 2016a) |
| H7N9 | LPAI | A/goose/Nebraska/17096-1/2011 | avian | 6 | 4 | (Belser et al., 2013a) |
| H7N9 | LPAI | A/shoveler/Egypt/00215-NAMRU3/2007 | avian | 6 | 3 | (Belser et al., 2013b) |
| H7N9 | LPAI | A/Anhui/1/2013 | Human fatal case | 14 | 12 | (Belser et al., 2016, 2013b) |
| H7N9 | LPAI | A/Shanghai/1/2013 | Human fatal case | 6 | 5 | (Belser et al., 2013b) |
| H7N9 | LPAI | A/Taiwan/1/2013 | Human respiratory case | 6 | 6 | (Belser et al., 2016) |
| H7N9 | LPAI | A/Hong Kong/5942/2013 | Human respiratory case | 6 | 6 | (Belser et al., 2016) |
| H7N9 | LPAI | A/British Columbia/1/2015 | Human respiratory case | 8 | 7 | (Belser et al., 2016) |
| H5N1 | HPAI | A/Hong Kong/483/1997 | Human fatal case | 12 | 9 | (Belser et al., 2009b) |
| H5N1 | HPAI | A/Hong Kong/486/1997 | Human respiratory case | 10 | 0 | (Belser et al., 2009b) |
| H5N1 | HPAI | A/Hong Kong/213/2003 | Human respiratory case | 6 | 0 | (Belser et al., 2009b) |
| H5N1 | HPAI | A/Vietnam/1203/2004 | Human fatal case | 10 | 4 | (Belser et al., 2009b) |
| H5N1 | HPAI | A/Thailand/16/2004 | Human fatal case | 12 | 9 | (Belser et al., 2009b) |
| H5N1 | HPAI | A/Indonesia/5/2005 | Human fatal case | 6 | 1 | this study |
| H3N2 | N/A | A/Aichi/1/1968 | Human respiratory case (seasonal) | 6 | 1 | (Belser et al., 2009b) |
| H3N2 | N/A | A/Memphis/102/1972 | Human respiratory case (seasonal) | 6 | 0 | (Belser et al., 2009b) |
| H1N1 | N/A | A/South Carolina/1/1918 | Reconstructed 1918 pandemic virus | 6 | 3 | (Belser et al., 2009b) |
| H1N1 | N/A | A/South Carolina/1/1918-AV | Reconstructed 1918 pandemic virus | 6 | 0 | (Belser et al., 2009b) |
| H1N1 | N/A | A/New Jersey/8/1976 | Human respiratory case (seasonal) | 6 | 0 | (Belser et al., 2010) |
| H1N1 | N/A | A/Ohio/2/2007 | Human respiratory case (triple reassortant swine) | 6 | 0 | (Belser et al., 2010) |
| H1N1 | N/A | A/California/4/2009 | Human respiratory case (2009 pandemic) | 6 | 0 | (Belser et al., 2011a) |
| H1N1 | N/A | A/California/4/2009-222G | Point mutant of 2009 pandemic virus | 6 | 0 | (Belser et al., 2011a) |
| H1N1 | N/A | A/Mexico/4108/2009 | Human respiratory case (2009 pandemic) | 6 | 0 | (Belser et al., 2010) |
| H1N1 | N/A | A/Mexico/4482/2009 | Human respiratory case (2009 pandemic) | 6 | 0 | (Belser et al., 2010) |
IVPI, intravenous pathogenicity index. HPAI, highly pathogenic avian influenza; LPAI, low pathogenic avian influenza. N/A, not applicable.
Total number of mice inoculated (inoc) by the ocular route with 106 PFU or EID50/5μl of virus.
Number of mice infected (inf) following ocular inoculation, defined by detection of infectious virus in the eye, nose, or lung through day 6 post inoculation.
Table 3.
Ocular infectivity of reassortant influenza viruses in mice.
| Donor virusa | Subtype | Day 3
|
Day 6
|
||||
|---|---|---|---|---|---|---|---|
| eye | nose | lung | eye | nose | lung | ||
| A/Puerto Rico/8/1934 | H1N1 | 0/5c | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| A/Vietnam/1203/2004b | H5N1 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| A/Indonesia/5/2005b | H5N1 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| A/Mexico/7218/2012 | H7N3 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| A/Anhui/1/2013 | H7N9 | 3/5 | 0/5 | 0/5 | 5/5 | 5/5 | 2/5 |
| A/Shanghai/2/2013 | H7N9 | 4/4 | 1/4 | 0/4 | 4/4 | 3/4 | 0/4 |
Reassortant viruses possessing the HA and NA of the strain indicated and internal genes from A/PR/8/34 virus.
The HA was modified by removal of the multibasic amino acid cleavage site.
Number of mice infected/total number of mice inoculated with 106 PFU or EID50/5 μl virus by the ocular route.
Reassortant viruses were generated by transfecting the reverse genetics plasmids encoding the HA and NA surface genes from the virus of interest along with the six internal genes from PR8 influenza virus into qualified Vero or 293-T cells (O’Neill and Donis, 2009; Ridenour et al., 2015; Sun et al., 2016b). For hemagglutinin genes derived from H5N1 highly pathogenic avian influenza virus, including A/Vietnam/1203/2004 and A/Indonesia/5/2005, overlapping PCR mutagenesis was performed to remove the polybasic amino acids (O’Neill and Donis, 2009; World Health Organization Global Influenza Program Surveillance, 2005).
2.2. Murine ocular inoculation
Female BALB/c mice (6-to-8 weeks of age) were inoculated by the ocular route as previously described (Belser et al., 2009b). Infection was determined by harvesting the eyes (pooled), nose, and lungs of mice on days 3 and 6 post-inoculation (p.i.), followed by homogenizing, and titrating them in eggs or cells. The limit of detection was 100.8 EID50/ml or 10 PFU/ml. Ocular pathotyping data from mice were collected from previously published studies (indicated with a reference in Table 1) or generated for this study (indicated as such in Table 1, Table 3). All wild-type viruses included in the study have been shown previously to infect mice by the intranasal inoculation route without prior adaptation. The dataset presented in Table 1 is limited to experiments performed at the Centers for Disease Control and Prevention under an Institutional Animal Care and Use Committee-approved protocol to minimize experimental variability.
2.3. Statistical analyses
Statistical analyses were performed using R version 3.2.3, including the binom and MRCV packages (Dorai-Raj, 2014; Koziol and Bilder, 2014; The R Core Team, 2015). Because multiple hypotheses were being tested, two-tailed p values < 0.01 are considered significant and 99% confidence intervals are given. Infection frequencies were compared using Fisher’s exact or Fisher-Freeman-Halton tests. Data comparing rates of virus detection in different tissues between H5 and H7 viruses were tested for simultaneous pairwise marginal independence using a modified Pearson chi-square statistic with Bonferroni adjustment as previously described (Agresti and Liu, 1999). Hemagglutinin subtype was treated as a single-response multiple-choice variable, while the tissues in which virus was detected was treated as a multiple-choice multiple response variable. Graphics were generated using GraphPad Prism 6 software.
3. Results
3.1. Contribution of surface glycoproteins to ocular infectivity
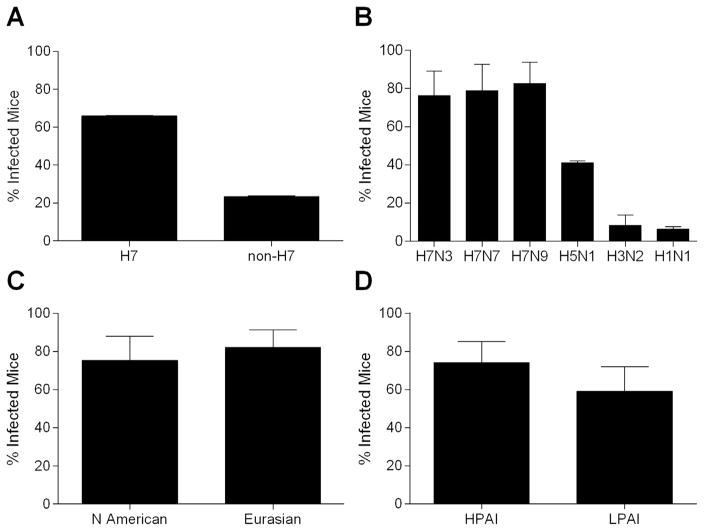
Influenza viruses of multiple subtypes have been shown to infect mice by the ocular route, with select H7 and H5 viruses capable of causing a fatal infection following ocular inoculation (Belser et al., 2009b). However, while H7 influenza viruses appear well-suited to infect mice by this inoculation route, it was unknown if the presence of an H7 hemagglutinin confers an enhanced ability for influenza virus to infect mice following ocular inoculation. To ascertain if murine infection following ocular inoculation occurred more frequently for viruses bearing an H7 hemagglutinin than for those of other HA subtypes, we examined a panel of 34 influenza viruses (Table 1). In this analysis, animals were counted as “infected” if infectious virus was detected in any tissue (eye, nose, or lung) above our limit of detection on either day 3 or 6 p.i. We found that H7 viruses were 2.8 times as likely to cause infection as were non-H7 viruses (p < 0.001) (Table 2, Fig. 1A). Separating the non-H7 viruses by subtype revealed that infection was most frequent after exposure to H5 viruses (41% of mice infected), but still lower than for H7 viruses (66% of mice infected, p < 0.001) (Table 2, Fig. 1B).
Table 2.
Frequency of infection following murine ocular inoculation by virus subtype.
| Virus subtypea | # of viruses | # infected mice | # mice inoculated | % Infected | Risk ratiob | p-valuec |
|---|---|---|---|---|---|---|
| H7 | 18 | 116 | 176 | 66% | – | – |
| Non-H7 | 16 | 27 | 116 | 23% | 2.8 | 2E–17 |
| H1 | 8 | 3 | 48 | 6% | 10.5 | 3E–19 |
| H3 | 2 | 1 | 12 | 8% | 7.9 | 2E–04 |
| H5 | 6 | 23 | 56 | 41% | 1.6 | 4E–05 |
Non-H7, inclusive of all viruses listed in Table 1.
Risk ratio for H7, using the indicated subtype as a reference.
p-value determined by Fisher’s exact test.
Fig. 1.
Risk of murine infection following ocular inoculation with influenza virus. Mice were inoculated by the ocular route with influenza viruses shown in Table 1 and separated into “infected” or “not infected” groups based on detection of infectious virus in the eye, nose, or lung through day 6 p.i. (limit of virus detection, 100.8 EID50/ml or 10 PFU/ml). Error bars represent 99% confidence intervals. A, risk of murine infection between all influenza viruses listed in Table 1 bearing an H7 hemagglutinin (18 viruses) and viruses bearing a non-H7 hemagglutinin (16 viruses) (p < 0.001). B, risk of murine infection between influenza viruses bearing the surface glycoproteins shown (4 H7N3, 3 H7N7, 7 H7N9, 6 H5N1, 2 H3N2, 8 H1N1 viruses), statistical information provided in Table 2. C, risk of murine infection between North American lineage (9 viruses; n=61 inoculated mice) or Eurasian lineage (9 viruses; n=84 inoculated mice) H7N3, H7N7, and H7N9 viruses (p > 0.01). D, risk of murine infectivity between H7 HPAI (6 viruses; n=84 inoculated mice) or LPAI (12 viruses; n=92 inoculated mice) influenza viruses listed in Table 1 (p > 0.01).
The plasticity of the H7 hemagglutinin to accommodate numerous neuraminidase subtypes in isolates associated with human infection is a feature not typically observed with other hemagglutinins. While ocular complications (with or without respiratory involvement) have been reported following human exposure with H7N2, H7N3, and H7N7 viruses, H7N9 viruses are typically associated with severe respiratory and not ocular disease (Belser et al., 2009a; To et al., 2014). To examine if the neuraminidase paired with the H7 hemagglutinin influences the capacity to cause infection following ocular inoculation in mice, we examined the risk of murine infection following inoculation with H7 viruses separated by NA subtype. We found that the neuraminidase coupled with the H7 HA did not significantly alter virus infectivity in this model, as the three H7 HA-NA combinations most frequently associated with human infection (H7N3, H7N7, and H7N9) all possessed infection rates > 75% with no significant differences observed among any of them (p=0.73, Fig. 1B; H7N2 and H7N8 were excluded from this analysis due to small sample size and lack of diversity among tested isolates). Both Eurasian and North American lineage H7 viruses have been associated with human infection, whereas human infections with H5N1 viruses have been limited to those from the Eurasian lineage. Among these three H7 HA-NA combinations, we did not detect a significant difference between North American (H7N3, H7N9) and Eurasian (H7N7, H7N9) lineage viruses in the frequency of ocular infection of mice (p=0.41, Fig. 1C). Collectively, these analyses indicate that the presence of an H7 hemagglutinin strongly affects the frequency of infection following ocular inoculation in the mouse model, regardless of the neuraminidase with which it is paired or the lineage from which it is derived.
3.2. Contribution of hemagglutinin cleavage site to ocular infectivity
Avian influenza viruses are classified as either highly pathogenic avian influenza (HPAI) or low pathogenic avian influenza (LPAI) viruses based on their virulence in chickens [per the Intravenous Pathogenicity Index (IVPI) or chicken lethality tests] or similarity of molecular attributes within the HA cleavage site motif to other HPAI viruses (OIE, 2015). While HPAI viruses frequently present with enhanced virulence in mammals compared with LPAI viruses, this is not always the case; both H7 HPAI and LPAI viruses have been associated with severe human infection and ocular disease. In mice, H7 HPAI viruses caused infection more frequently than did LPAI viruses (74% vs 59%, respectively), but this difference was not statistically significant (Fig. 1D). These results affirm that H7 LPAI and HPAI viruses are both capable of causing mammalian infection by the ocular route.
Among H7 viruses, it is not uncommon for a LPAI virus to acquire a HPAI phenotype during the course of an outbreak in poultry; examination of both LPAI and HPAI viruses sourced from the same outbreak thus permits an opportunity to examine specific molecular determinants of pathogenicity that may not be possible among other wild-type viruses. To more closely investigate the role of the HA cleavage site in ocular infectivity, we examined two pairs of viruses isolated from the same outbreak that emerged via nonhomologous recombination events and differ primarily in their IVPI classification and cleavage site. The H7N3 viruses A/Canada/504/2004 and A/Canada/444/2004 share high sequence identity and differ in their HA cleavage site by one amino acid (Hirst et al., 2004), and the H7N8 viruses A/turkey/Indiana/1403/2016 and A/turkey/Indiana/1573-2/2016 differ by only eight amino acids and a three basic residue insertion in the HA cleavage site (Sun et al., 2016a). As shown in Table 1, we observed no pronounced difference in murine infectivity between either pair of viruses (76% and 83% infectivity between H7N3 HPAI and LPAI viruses and 11% and 0% infectivity between H7N8 HPAI and LPAI viruses, respectively), further supporting a noncritical role for chicken virulence classification and cleavage site composition in mammalian ocular infectivity (Belser et al., 2009b; Sun et al., 2016a).
3.3. Contribution of internal genes to ocular infectivity
Despite the elevated frequency of infection following ocular inoculation with H7 viruses compared with non-H7 viruses, the presence of an H7 HA alone is not sufficient to confer an ocular tropism in mice or humans (Belser et al., 2013c). As such, it is likely that internal virus genes contribute to this property. To more specifically address this question, we examined the infectivity of reassortant influenza viruses possessing surface glycoproteins from wild-type influenza viruses with all internal genes derived from A/Puerto Rico/8/1934 (PR/8), a laboratory-adapted H1N1 virus found not to infect mice by the ocular route (Table 3). Infectivity in mice by the ocular route was maintained despite replacing the internal genes of the H7N9 LPAI virus A/Anhui/1/2013, at 86% (following wild-type virus infection, Table 1) and 80% (following PR/8 reassortant virus infection, Table 3). In support of this finding, an additional H7N9 PR/8 reassortant virus tested, bearing surface glycoproteins from A/Shanghai/2/2013 virus, exhibited 100% infectivity in mice by the ocular route (Table 3). However, replacing the internal genes of the H7N3 HPAI virus A/Mexico/InDRE7218/2012 was sufficient to abrogate virus infectivity by the ocular route in mice, from 94% (following wild-type virus infection, Table 1), to 0% (following PR/8 reassortant virus infection, Table 3). These findings indicate that internal genes likely contribute to ocular tropism in a strain-specific manner.
The amino acid position at 627 in PB2 has been identified as a molecular determinant of host range and virulence for influenza viruses, including H7 subtype viruses (Munster et al., 2007; Subbarao et al., 1993). Many H5N1 and H7N9 influenza viruses associated with human infection contain an E627K mutation in PB2 and possess enhanced virulence in small mammalian models (mouse and ferret), in contrast with many ocular-tropic H7 subtype viruses associated with human infection, which generally retain a glutamic acid at position 627 (Fouchier et al., 2004). To examine the role of this amino acid in murine ocular infectivity, we compared the risk of infection following ocular inoculation between viruses with a lysine and those with a glutamic acid residue at this position (Fig. 2). Among all viruses identified in Table 1, 627K was associated with a significantly higher risk of infection in mice compared with 627E (64% vs 37% respectively, p < 0.001). When viruses were separated by subtype, 627K was associated with a higher risk of infection for both H7 and non-H7 viruses (p < 0.002 for both, Fig. 2A and B). This association was maintained when H7 subtype viruses were separated into H7N9-only and non-H7N9 groups, indicating that the association between 627K and increased risk of infection was not confounded by the presence of several 627K-bearing-bearing H7N9 viruses in our analysis (data not shown). Interestingly, this analysis revealed that while both 627K and 627E H7 subtype viruses were associated with murine infection following ocular inoculation, only non-H7 subtype viruses possessing a lysine at position 627 were associated with murine infection (Fig. 2B). This suggests that the residue at position 627 may have a greater influence on the ability of non-H7 viruses to infect via the ocular route compared to H7 viruses. However, logistic regression did not find the interaction between having an H7 HA and PB2 627K significant, likely due to the low power of this analysis.
Fig. 2.
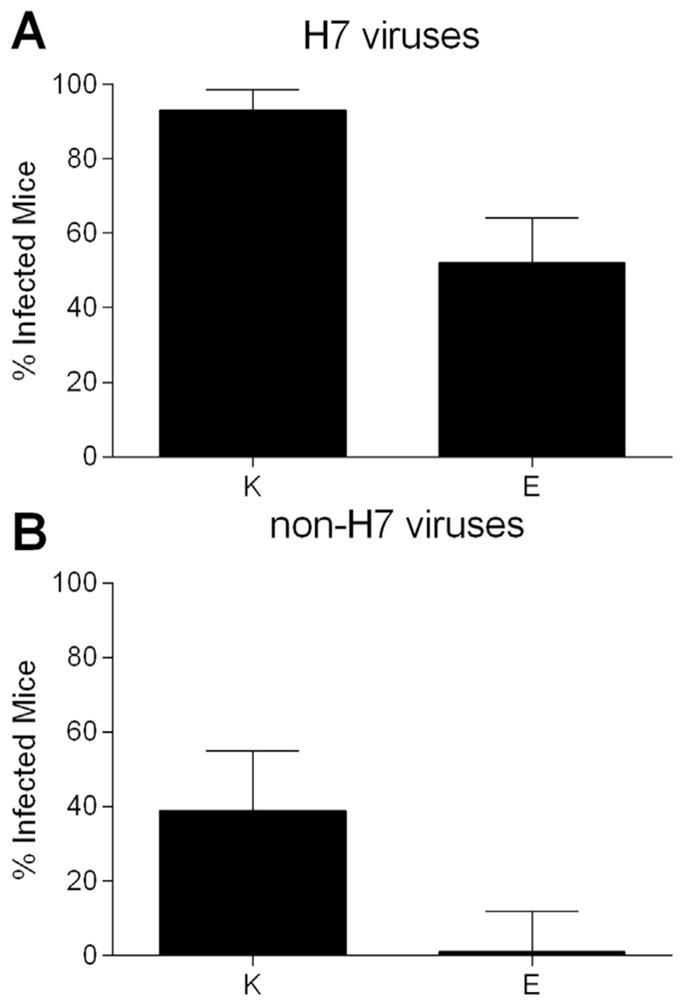
Contribution of PB2 627 to risk of murine infection following ocular inoculation with influenza virus. Mice were inoculated by the ocular route with influenza viruses shown in Table 1 and separated into “infected” or “not infected” groups based on detection of infectious virus in the eye, nose, or lung through day 6 p.i. (limit of virus detection, 100.8 EID50/ml or 10 PFU/ml). Error bars represent 99% confidence intervals. Relative risk of murine infection between influenza viruses bearing a lysine (K) or glutamic acid (E) at position 627 among A, all H7 viruses (6 627K viruses, n=60 inoculated mice; 12 627E viruses, n=116 inoculated mice) or B, non-H7 viruses (9 627K viruses, n=70 inoculated mice; 7 627E viruses, n=46 inoculated mice), p < 0.002 for both comparisons.
3.4. Subtype-specific tropism following ocular inoculation
Due in part to replication-independent drainage of virus from the eye to nasopharyngeal space, influenza virus can be detected in both ocular and respiratory tract tissues following ocular inoculation (Belser et al., 2012a). After finding that H7 subtype viruses are statistically more likely to cause infection in mice following ocular inoculation compared with H5 viruses, we next investigated more specifically the frequency in which infectious virus is detected in the eye, nose, and lung of mice inoculated by the ocular route with ocular-tropic (H7 subtype) or respiratory-tropic (H5 subtype) influenza viruses. Among mice that were infected following ocular inoculation with either wild-type H5 or H7 subtype viruses, we found that H7 subtype viruses were significantly more likely to be detected in the eye or nose through day 6 p.i. than were H5 subtype viruses (p < 0.001 and p < 0.01, respectively), with > 70% of H7 virus-infected mice possessing detectable virus in both tissues (75% and 72%, respectively) (Fig. 3). In contrast, mice infected with H5 viruses more frequently possessed detectable virus in the nose (61%) than eye (26%), mirroring the respiratory tropism of this subtype in humans. Rates of virus detection in the lung were not significantly different between these virus subtypes (41% and 35% for H7 and H5 subtype viruses, respectively). Viral titers detected in these samples were generally low ( < 3 log10 PFU or EID50/ml) independent of virus subtype (data not shown), suggesting that this murine model of ocular inoculation is most suitable for examining frequency of detection of infectious virus, rather than the magnitude of the detected titer. These analyses reveal that H7 subtype viruses not only infect mice at a higher rate following ocular inoculation than other virus subtypes, but also indicate that they are more likely to replicate in ocular tissue during the acute phase of infection.
Fig. 3.
Differential detection of infectious virus following ocular inoculation in mice. Mice were inoculated by the ocular route with H5 and H7 subtype influenza viruses shown in Table 1. The risk of positive virus detection in the eye, nose, or lung through day 6 p.i. based on virus subtype is shown (limit of virus detection, 100.8 EID50/ml or 10 PFU/ml). Error bars represent 99% confidence intervals. H5: 6 viruses, n=23 inoculated mice per tissue. H7: 18 viruses, n=114 (eye) or 116 (nose, lung) inoculated mice. Statistical significance between H7 and H5 subtype viruses is p < 0.001 (eye), p < 0.01 (nose), p > 0.01 (lung).
4. Discussion
H7 subtype influenza viruses typically exhibit an ocular tropism in humans, causing conjunctivitis with or without associated respiratory involvement. In this regard, influenza virus is similar to other principal respiratory viruses (such as respiratory syncytial virus and adenovirus), for which a particular subtype or subgroup appears to possess a non-respiratory tropism. There is a poor understanding of what contributes to the non-respiratory tropism of all of these viruses (Belser et al., 2013c). Well-characterized laboratory models which recapitulate a tropism detected in humans represent an essential tool to elucidate the viral and host factors which confer this property. Our study identifies murine ocular inoculation as the only mammalian model characterized to date which mirrors the apparent ocular tropism associated with H7 virus subtype infection in humans.
Murine models for ocular inoculation and ocular infection have been characterized for several respiratory pathogens (Belser et al., 2013c). Despite the utility of these models to study ocular exposure to virus, these species do not fully emulate the ocular disease observed in humans. In particular, while conjunctivitis is the most frequently reported ocular complication associated with influenza virus infection, neither mice nor ferrets typically display macroscopic signs of conjunctivitis. While the lack of clinical presentation of ocular symptoms limits our full capacity to study the ocular tropism of selected respiratory viruses, these models nonetheless provide the ability to better understand the capacity and propensity for ocular infection and ocular replication.
Epithelia of the human cornea and surrounding conjunctiva bear a predominance of α2–3 linked sialic acids (the cellular receptor preferred by most avian influenza viruses), and a paucity of α2–6 linked sialic acids (preferred by most human influenza viruses) (Kumlin et al., 2008). Thus, it has been postulated that avian influenza viruses may be better suited to infecting the α2–3-rich ocular environment (Olofsson et al., 2005). However, we showed previously via virus immunohistochemistry that both human and avian influenza viruses can attach to murine corneal tissue, and further demonstrated productive virus replication of both H5 and H7 influenza viruses in excised murine whole corneas or corneal epithelial sheets (Belser et al., 2009b). Furthermore, many H5N1 viruses tested that exhibit strong binding to α2–3 sialosides were not capable of causing robust infection following ocular inoculation in mice (including A/Hong Kong/486/1997 and A/Indonesia/5/2005) (Belser et al., 2009b; Stevens et al., 2008), whereas H7 subtype viruses with both strict α2–3 (including A/Netherlands/219/2003) and mixed α2–3/α2–6 (including A/Canada/504/2004) binding properties demonstrated a high capacity for infectivity following ocular exposure (Belser et al., 2008). While receptor-binding differences may yet contribute to ocular infectivity, these data suggest that an α2–3 binding preference alone is insufficient to confer an ocular tropism to a virus which otherwise does not possess this capacity. Further detailed characterization of the human ocular surface, especially with regard to sialoside composition and density, is needed, as is a greater understanding of conjunctivitis and/or other ocular complications following human exposure to avian compared with human influenza viruses.
We found that among H7N3, H7N7, and H7N9 viruses, murine infectivity following ocular inoculation was independent of neuraminidase pairing or virus lineage. However, it is important to note that in these two specific analyses, H7N2 and H7N8 pairings were excluded due to low representation (n=2 unique viruses for each) and diversity in our dataset. It is unclear if the lack of infectivity detected among these viruses (North American avian isolates from 2002–3 [H7N2] or 2016 [H7N8]) following ocular inoculation in mice is truly representative of these HA-NA combinations or rather a reflection of poor diversity in available viruses to include for this analysis and lack of Eurasian lineage viruses to include for comparison. It should be noted that Eurasian lineage, but not North American lineage H7N2 viruses, have been associated with ocular disease in humans (Belser et al., 2009a). Further investigation of these viruses, as well as other H7 HA-NA pairings causing poultry outbreaks (such as H7N1) will permit a greater understanding of the propensity for ocular disease.
H7 subtype influenza viruses associated with ocular disease in humans represent a wide range of heterogeneity, including variance within the neuraminidase subtype, virus lineage, and pathotype. Notably, H7 viruses associated with human conjunctivitis have acquired a HPAI phenotype by the traditional insertion of multiple basic amino acids at the HA cleavage site, or by nonhomologous recombination with other viral gene segments or host ribosomal RNA (Hirst et al., 2004; Lopez-Martinez et al., 2013; Pasick et al., 2005), underscoring a diversity of cleavage site compositions. However, H7 LPAI viruses bearing a single basic amino acid at the HA cleavage site have also been associated with ocular disease in humans and murine ocular infection (Belser et al., 2009a). To further support that ocular tropism is not affected by cleavage site, we found that H7N9-PR8 reassortant viruses, but not H5N1-PR8 reassortant viruses, for which the multibasic amino acid cleavage site was removed to match the single arginine cleavage site composition of H7N9 viruses, were infectious following ocular inoculation in mice (Table 3). Collectively, this study confirms that intrinsic properties of the H7 hemagglutinin, independent of cleavage site, confer an enhanced ability to infect following ocular exposure in mammals.
Compared with other H7 viruses, H7N9 influenza viruses associated with human infection more closely resemble H5N1 viruses with regard to their respiratory tropism, elicitation of innate host responses, and capacity to cause severe respiratory disease in humans (Chan et al., 2013; Zeng et al., 2015). Despite an absence of ocular tropism with this virus subtype, H7N9 viruses from 2013 to 2015 nonetheless appear to possess the capacity for murine infectivity following ocular inoculation (Fig. 1), and have been detected in ocular secretions from intranasally inoculated mice (Bao et al., 2014). It is currently unclear what governs this altered tissue tropism of H7N9 viruses in humans. Previous study has identified a synergistic role for both HA and NA genes from a 2009 H1N1 virus in conferring conjunctival tropism in vitro (Chan et al., 2011), identifying a need to further study in additional laboratory models the role N9 neuraminidase may have in influencing this property.
The role of internal genes in the ocular tropism of H7 influenza viruses is not clear, and represents an area of much-needed research. Our finding that the surface glycoproteins are not sufficient to maintain the infectivity of an H7N3 HPAI virus by the ocular route (Table 3) identifies a potential role for internal proteins in this property, warranting further study to identify the specific gene(s) responsible for this result. It is possible that the replication advantage among H7N9 and H5N1 viruses bearing E627K in PB2, demonstrated in both avian and mammalian cells (Yamayoshi et al., 2015; Zhang et al., 2014), permits enhanced infectivity by the ocular route in mice. H7N7 and H5N1 viruses bearing the 627K substitution have replicated to higher titer than 627E-bearing viruses in human corneal and conjunctival cells in vitro (Belser et al., 2011b). However, studies identifying that an E627K mutation alone do not uniformly confer higher growth rates and virulence in mammalian models, and our finding that H7 subtype viruses bearing either 627E and 627K can infect mice following ocular inoculation (Fig. 2), suggests that this substitution alone is neither necessary nor sufficient (Zhu et al., 2010). Clearly, there remains a need for a better understanding of molecular determinants of non-respiratory tropism of influenza virus.
The human eye is a complex organ, with several tissue components exposed to the environment and thus susceptible to exposure to virus infection by aerosol or fomites. As no one mammalian model or in vitro system can fully recapitulate the diversity of the human ocular milieu, the study of complex virus-host interactions at the ocular surface poses an ongoing challenge in the laboratory. The murine model of ocular inoculation described here currently represents the most stringent in vivo model available to study the ocular tropism possessed by H7 subtype viruses. The use of models such as this has permitted the investigation of antiviral drugs to mitigate viral replication and spread following H7 ocular exposure (Belser et al., 2012b), underscoring the need for well-characterized mammalian models to examine the diversity of influenza virus exposure routes and disease presentations.
Acknowledgments
The authors thank members of the international scientific community and the WHO Global Influenza Surveillance and Response System (GISRS) for facilitating access to many of the viruses used in this study. HMC is supported by the Oak Ridge Institute for Science and Education. The findings and conclusions are those of the authors and do not necessarily reflect the views of the funding agency.
References
- Aamir UB, Naeem K, Ahmed Z, Obert CA, Franks J, Krauss S, Seiler P, Webster RG. Zoonotic potential of highly pathogenic avian H7N3 influenza viruses from Pakistan. Virology. 2009;390:212–220. doi: 10.1016/j.virol.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti A, Liu IM. Modeling a categorical variable allowing arbitrarily many category choices. Biometrics. 1999;55:936–943. doi: 10.1111/j.0006-341x.1999.00936.x. [DOI] [PubMed] [Google Scholar]
- Bao L, Xu L, Zhu H, Deng W, Chen T, Lv Q, Li F, Yuan J, Xu Y, Huang L, Li Y, Liu J, Yao Y, Yu P, Chen H, Qin C. Transmission of H7N9 influenza virus in mice by different infective routes. Virol J. 2014;11:185. doi: 10.1186/1743-422X-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, Donis R, Busch J, McBride R, Paulson JC, Katz JM, Tumpey TM. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc Natl Acad Sci USA. 2008;105:7558–7563. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Bridges CB, Katz JM, Tumpey TM. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg Infect Dis. 2009a;15:859–865. doi: 10.3201/eid1506.090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Creager HM, Sun X, Gustin KM, Jones T, Shieh WJ, Maines TR, Tumpey TM. Mammalian pathogenesis and transmission of H7N9 influenza viruses from three waves, 2013–2015. J Virol. 2016;90:4647–4657. doi: 10.1128/JVI.00134-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Davis CT, Balish A, Edwards LE, Zeng H, Maines TR, Gustin KM, Martinez IL, Fasce R, Cox NJ, Katz JM, Tumpey TM. Pathogenesis, transmissibility, and ocular tropism of a highly pathogenic avian influenza A (H7N3) virus associated with human conjunctivitis. J Virol. 2013a;87:5746–5754. doi: 10.1128/JVI.00154-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Gustin KM, Katz JM, Maines TR, Tumpey TM. Influenza virus infectivity and virulence following ocular-only aerosol inoculation of ferrets. J Virol. 2014;88:9647–9654. doi: 10.1128/JVI.01067-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Gustin KM, Maines TR, Pantin-Jackwood MJ, Katz JM, Tumpey TM. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathog. 2012a;8:e1002569. doi: 10.1371/journal.ppat.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, Sun X, Carney PJ, Villanueva JM, Stevens J, Katz JM, Tumpey TM. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. 2013b;501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Jayaraman A, Raman R, Pappas C, Zeng H, Cox NJ, Katz JM, Sasisekharan R, Tumpey TM. Effect of D222G mutation in the hemagglutinin protein on receptor binding, pathogenesis and transmissibility of the 2009 pandemic H1N1 influenza virus. PLoS One. 2011a;6:e25091. doi: 10.1371/journal.pone.0025091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Rota PA, Tumpey TM. Ocular tropism of respiratory viruses. Microbiol Mol Biol Rev. 2013c;77:144–156. doi: 10.1128/MMBR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Sleeman K, Pearce MB, Katz JM, Gubareva LV, Tumpey TM. Oseltamivir inhibits H7 influenza virus replication in mice inoculated by the ocular route. Antimicrob Agents Chemother. 2012b;56:1616–1618. doi: 10.1128/AAC.06101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Wadford DA, Pappas C, Gustin KM, Maines TR, Pearce MB, Zeng H, Swayne DE, Pantin-Jackwood M, Katz JM, Tumpey TM. Pathogenesis of pandemic influenza A (H1N1) and triple-reassortant swine influenza A (H1) viruses in mice. J Virol. 2010;84:4194–4203. doi: 10.1128/JVI.02742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Wadford DA, Xu J, Katz JM, Tumpey TM. Ocular infection of mice with influenza A (H7) viruses: a site of primary replication and spread to the respiratory tract. J Virol. 2009b;83:7075–7084. doi: 10.1128/JVI.00535-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Zeng H, Katz JM, Tumpey TM. Ocular tropism of influenza A viruses: identification of H7 subtype-specific host responses in human respiratory and ocular cells. J Virol. 2011b;85:10117–10125. doi: 10.1128/JVI.05101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MC, Chan RW, Chan LL, Mok CK, Hui KP, Fong JH, Tao KP, Poon LL, Nicholls JM, Guan Y, Peiris JS. Tropism and innate host responses of a novel avian influenza A H7N9 virus: an analysis of ex-vivo and in-vitro cultures of the human respiratory tract. Lancet Respir Med. 2013;1:534–542. doi: 10.1016/S2213-2600(13)70138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MC, Chan RW, Yu WC, Ho CC, Yuen KM, Fong JH, Tang LL, Lai WW, Lo AC, Chui WH, Sihoe AD, Kwong DL, Wong DS, Tsao GS, Poon LL, Guan Y, Nicholls JM, Peiris JS. Tropism and innate host responses of the 2009 pandemic H1N1 influenza virus in ex vivo and in vitro cultures of human conjunctiva and respiratory tract. Am J Pathol. 2010;176:1828–1840. doi: 10.2353/ajpath.2010.091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RW, Kang SS, Yen HL, Li AC, Tang LL, Yu WC, Yuen KM, Chan IW, Wong DD, Lai WW, Kwong DL, Sihoe AD, Poon LL, Guan Y, Nicholls JM, Peiris JS, Chan MC. Tissue tropism of swine influenza viruses and reassortants in ex vivo cultures of the human respiratory tract and conjunctiva. J Virol. 2011;85:11581–11587. doi: 10.1128/JVI.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chosewood LC, Wilson DE Centers for Disease Control and Prevention (U.S.), National Institutes of Health (U.S.) Biosafety in microbiological and biomedical laboratories. 5. U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institutes of Health; Washington, D.C: 2009. [Google Scholar]
- Dorai-Raj S. Binom: Binomial Confidence Intervals for Several Parameterizations. 2014. [Google Scholar]
- Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci USA. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M, Astell CR, Griffith M, Coughlin SM, Moksa M, Zeng T, Smailus DE, Holt RA, Jones S, Marra MA, Petric M, Krajden M, Lawrence D, Mak A, Chow R, Skowronski DM, Tweed SA, Goh S, Brunham RC, Robinson J, Bowes V, Sojonky K, Byrne SK, Li Y, Kobasa D, Booth T, Paetzel M. Novel avian influenza H7N3 strain outbreak, British Columbia. Emerg Infect Dis. 2004;10:2192–2195. doi: 10.3201/eid1012.040743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol N, Bilder C. MRCVs. 2014. MRCV: methods for analyzing multiple response categorical variables. [Google Scholar]
- Kumlin U, Olofsson S, Dimock K, Arnberg N. Sialic acid tissue distribution and influenza virus tropism. Influenza Other Respir Virus. 2008;2:147–154. doi: 10.1111/j.1750-2659.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Martinez I, Balish A, Barrera-Badillo G, Jones J, Nunez-Garcia TE, Jang Y, Aparicio-Antonio R, Azziz-Baumgartner E, Belser JA, Ramirez-Gonzalez JE, Pedersen JC, Ortiz-Alcantara J, Gonzalez-Duran E, Shu B, Emery SL, Poh MK, Reyes-Teran G, Vazquez-Perez JA, Avila-Rios S, Uyeki T, Lindstrom S, Villanueva J, Tokars J, Ruiz-Matus C, Gonzalez-Roldan JF, Schmitt B, Klimov A, Cox N, Kuri-Morales P, Davis CT, Diaz-Quinonez JA. Highly pathogenic avian influenza A(H7N3) virus in poultry workers, Mexico, 2012. Emerg Infect Dis. 2013;19:1531–1534. doi: 10.3201/eid1909.130087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Geiler J, Klassert D, Doerr HW, Cinatl J., Jr Infection of human retinal pigment epithelial cells with influenza A viruses. Investig Ophthalmol Vis Sci. 2009;50:5419–5425. doi: 10.1167/iovs.09-3752. [DOI] [PubMed] [Google Scholar]
- Munster VJ, de Wit E, van Riel D, Beyer WE, Rimmelzwaan GF, Osterhaus AD, Kuiken T, Fouchier RA. The molecular basis of the pathogenicity of the Dutch highly pathogenic human influenza A H7N7 viruses. J Infect Dis. 2007;196:258–265. doi: 10.1086/518792. [DOI] [PubMed] [Google Scholar]
- O’Neill E, Donis RO. Generation and characterization of candidate vaccine viruses for prepandemic influenza vaccines. Curr Top Microbiol Immunol. 2009;333:83–108. doi: 10.1007/978-3-540-92165-3_4. [DOI] [PubMed] [Google Scholar]
- OIE. Avian Influenza (Infection with avian influenza viruses) 2015 Chapter 2. 3.4. < http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.03.04_AI.pdf>.
- Olofsson S, Kumlin U, Dimock K, Arnberg N. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect Dis. 2005;5:184–188. doi: 10.1016/S1473-3099(05)01311-3. [DOI] [PubMed] [Google Scholar]
- Pasick J, Handel K, Robinson J, Copps J, Ridd D, Hills K, Kehler H, Cottam-Birt C, Neufeld J, Berhane Y, Czub S. Intersegmental recombination between the haemagglutinin and matrix genes was responsible for the emergence of a highly pathogenic H7N3 avian influenza virus in British Columbia. J Gen Virol. 2005;86:727–731. doi: 10.1099/vir.0.80478-0. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench HA. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Ridenour C, Johnson A, Winne E, Hossain J, Mateu-Petit G, Balish A, Santana W, Kim T, Davis C, Cox NJ, Barr JR, Donis RO, Villanueva J, Williams TL, Chen LM. Development of influenza A(H7N9) candidate vaccine viruses with improved hemagglutinin antigen yield in eggs. Influenza Other Respir Virus. 2015;9:263–270. doi: 10.1111/irv.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Blixt O, Chen LM, Donis RO, Paulson JC, Wilson IA. Recent avian H5N1 viruses exhibit increased propensity for acquiring human receptor specificity. J Mol Biol. 2008;381:1382–1394. doi: 10.1016/j.jmb.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao EK, London W, Murphy BR. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Luo J, Gao Y, He H. Different infection routes of avian influenza A (H5N1) virus mice. Integr Zool. 2009;4:402–408. doi: 10.1111/j.1749-4877.2009.00178.x. [DOI] [PubMed] [Google Scholar]
- Sun X, Belser JA, Pulit-Penaloza JA, Zeng H, Lewis A, Shieh WJ, Tumpey TM, Maines TR. Pathogenesis and transmission assessments of two H7N8 influenza viruses recently isolated from turkey farms in Indiana using mouse and ferret models. J Virol. 2016a doi: 10.1128/JVI.01646-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Belser JA, Tumpey TM. A novel eight amino acid insertion contributes to the hemagglutinin cleavability and the virulence of a highly pathogenic avian influenza A (H7N3) virus in mice. Virology. 2016b;488:120–128. doi: 10.1016/j.virol.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock GA, Paul JA, Barry RD. Immunization against influenza by the ocular route. Vaccine. 1985;3:277–280. doi: 10.1016/0264-410x(85)90122-7. [DOI] [PubMed] [Google Scholar]
- To KK, Chan JF, Yuen KY. Viral lung infections: epidemiology, virology, clinical features, and management of avian influenza A(H7N9) Curr Opin Pulm Med. 2014;20:225–232. doi: 10.1097/MCP.0000000000000047. [DOI] [PubMed] [Google Scholar]
- The R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- World Health Organization Global Influenza Program Surveillance, N. Evolution of H5N1 avian influenza viruses in Asia. Emerg Infect Dis. 2005;11:1515–1521. doi: 10.3201/eid1110.050644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamayoshi S, Fukuyama S, Yamada S, Zhao D, Murakami S, Uraki R, Watanabe T, Tomita Y, Neumann G, Kawaoka Y. Amino acids substitutions in the PB2 protein of H7N9 influenza A viruses are important for virulence in mammalian hosts. Sci Rep. 2015;5:8039. doi: 10.1038/srep08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Belser JA, Goldsmith CS, Gustin KM, Veguilla V, Katz JM, Tumpey TM. A(H7N9) virus results in early induction of proinflammatory cytokine responses in both human lung epithelial and endothelial cells and shows increased human adaptation compared with avian H5N1 virus. J Virol. 2015;89:4655–4667. doi: 10.1128/JVI.03095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, Tumpey TM, Katz JM. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type i interferon response in polarized human bronchial epithelial cells. J Virol. 2007;81:12439–12449. doi: 10.1128/JVI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li X, Guo J, Li L, Chang C, Li Y, Bian C, Xu K, Chen H, Sun B. The PB2 E627K mutation contributes to the high polymerase activity and enhanced replication of H7N9 influenza virus. J Gen Virol. 2014;95:779–786. doi: 10.1099/vir.0.061721-0. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wang J, Wang P, Song W, Zheng Z, Chen R, Guo K, Zhang T, Peiris JS, Chen H, Guan Y. Substitution of lysine at 627 position in PB2 protein does not change virulence of the 2009 pandemic H1N1 virus in mice. Virology. 2010;401:1–5. doi: 10.1016/j.virol.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]