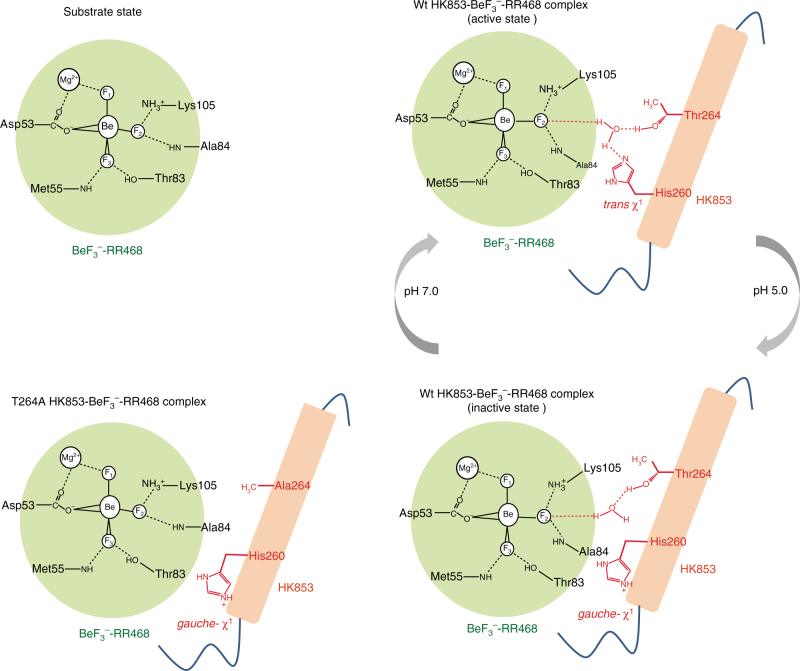
Fig. 3.
Proposed role of the conserved HxxxT motif in the pH-gated phosphatase activity of HisKA. Residues in the active site of RR468 and HK853 are shown in black and red, respectively. In the proposed model, the substrate state (upper left) defines the apo state fluorine chemical shifts, which reflects a lack of specific interactions of the F2 fluorine atom with HK853. In the catalytically active state (Fca state, upper right), the F2 fluorine atom (mimicking an oxygen atom of the phosphate group) forms a hydrogen bond with the catalytic water molecule, which is activated by T264 and H260 of HK853, with H260 in the trans χ 1 rotameric conformation poised for the catalytic attack of the phosphorus atom of phosphorylated D53 (mimicked by BeF3 −-D53) of RR468. Under acidic conditions, the protonated H260 swings away from the catalytic water molecule, leaving HK853 T264 as the only remaining residue interacting with the catalytic water molecule and the F2 fluorine atom of BeF3 –-RR468, and reflecting a catalytically inactive state of HK853 (Fci, lower right). The F2 atom of BeF3 --D53 in the T264A complex shows a similar chemical environment to the F2 apo state despite the formation of the T264A HK853-BeF3 --RR468 complex, consistent with the loss of the F2-interacting catalytic water molecule due to the change of H260 χ 1 rotameric conformation to gauche- and the elimination of the side chain hydroxyl group from the T264A mutation (lower left)