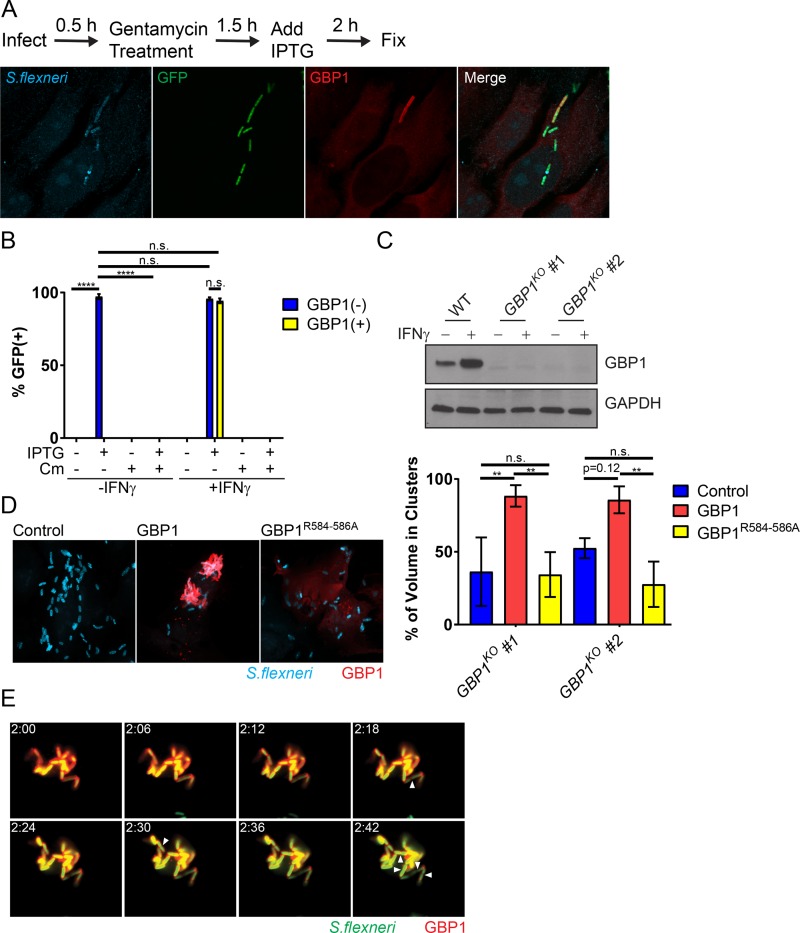
FIG 5 .
GBP1-tagged S. flexneri cells replicate within intracellular bacterial aggregates. (A) IFN-γ-primed and unprimed HeLa cells were infected at an MOI of 50 with S. flexneri carrying an IPTG-inducible GFP reporter plasmid. IPTG and, where indicated, also chloramphenicol (Cm) were added to the culture medium at 2 hpi. At 4 hpi, cells were fixed and stained with anti-LPS (blue) and anti-GBP1 (red). A representative image of infected, IFN-γ-primed HeLa cells is shown. (B) GFP expression was used as a proxy for bacterial viability. Combined data from 3 independent experiments as described for panel A are shown. (C) Protein lysates from IFN-γ-primed parental HeLa (WT) and two independent GBP1KO clones were transferred to membranes and probed with anti-GBP1 and anti-GAPDH antibodies. (D) Two independent GBP1KO HeLa cell clones were transfected with wild-type GBP1 or GBP1R584–586A mCherry fusion proteins and then infected with S. flexneri at an MOI of 50. Bacteria were visualized with anti-LPS (blue) immunostaining. Cluster analysis was performed as described in Materials and Methods, and the percentage of total bacterial volume occupied by clusters of greater than three bacteria was determined in mCherry-positive cells using the ImageJ 3D-OC plugin. The graph depicts the combined data from three independent experiments. Error bars represent SEM. (E) GBP1KO HeLa cells were transfected with mCherry-GBP1 and infected with poly-d-lysine-pretreated S. flexneri at an MOI of 10. Cells were infected for 30 min, washed, and then placed in phenol red-free DMEM. Video microscopy was begun at 2 hpi (2:00 a.m.). Images of 6-min intervals between 2:00 a.m. and 2:42 are shown. Arrowheads point to bacteria that have undergone cell division. Two-way ANOVA was used to assess statistical significance. ****, P < 0.0001; n.s., nonsignificant.