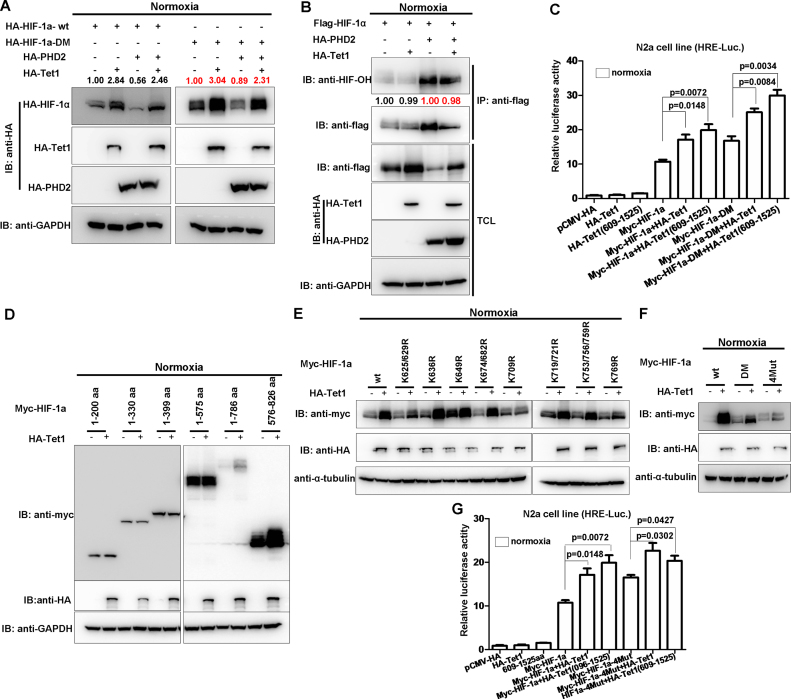
Figure 7.
Tet1 does not affect PHD2 function on HIF-1α and stabilizes the C-terminal of HIF-1α. (A) The hydroxylated site-mutated HIF-1α (HIF-1α DM) still was stabilized by Tet1. (B) Tet1 had no effect on hydroxylation of HIF-1α. HEK293T cells were transfected with the indicated plasmids. After co-immunoprecipitation using anti-flag conjugated agarose beads, the loading amount of protein was adjusted to the similar level between the samples with and without Tet1 overexpression based on the pilot experiments. (C) Overexpression of mouse Tet1 (tet1-wt) and its domain covering 609-1525aa enhanced the activity of the hydroxylated site mutated HIF-1α (HIF-1α DM) on the hypoxia response element luciferase (HRE-luc.) reporter in the N2a cell line under normoxia. (D) Tet1 stabilized the C-terminal of HIF-1α (576-826 aa). (E) Tet1 stabilized the different lysine mutants of HIF-1α. (F) The four-lysine site mutated HIF-1α (HIF-1α-4Mut) (K625/629/709/769R) still was stabilized by Tet1, but it was not as dramatic as that of wild-type HIF-1α or the hydroxylated site mutated HIF-1α (DM). (G) Overexpression of mouse Tet1 (tet1-wt) and its domain covering 609-1525aa could still enhance the activity of HIF-1α-4Mut on the HRE-luc. reporter in the N2a cell line under normoxia.