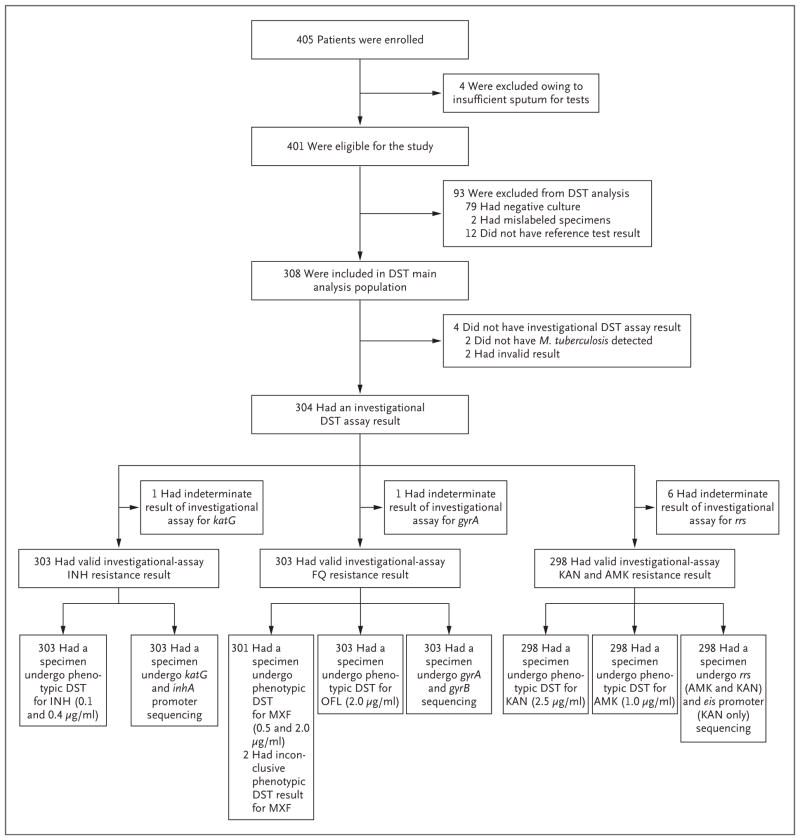
Figure 1. Participant Enrollment and Testing in the Main Analysis Population.
Among the 12 patients who were excluded from the analysis of drug-susceptibility testing (DST) because of a lack of a reference-test result, 10 had DNA that was of insufficient quality or quantity for sequencing, and 2 had uninterpretable MGIT phenotypic DST results because of contamination. Complete DST reference-standard results were not achievable for 4.3% of participants (14 of 322) whose culture was positive for Mycobacterium tuberculosis. Among the 308 participants in the main analysis population for DST, 152 were excluded from the reflex-test analysis population (146 with an Xpert MTB/RIF result indicating that rifampin resistance was not detected, 4 with an Xpert MTB/RIF result indicating that M. tuberculosis was not detected, and 2 with an indeterminate Xpert MTB/RIF result with regard to rifampin resistance); the reflex-test analysis population therefore included 156 participants. AMK denotes amikacin, FQ fluoroquinolone, INH isoniazid, KAN kanamycin, MXF moxifloxacin, and OFL ofloxacin.