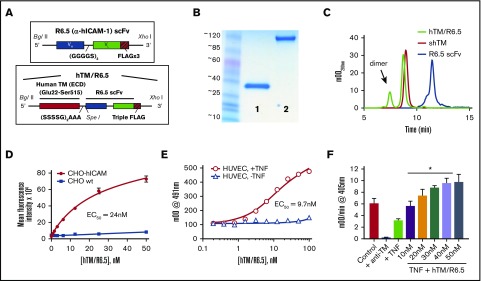
Figure 1.
Assembly, characterization, and functional activity of hTM/R6.5 biotherapeutic. (A) Molecular design of R6.5 scFv and hTM/R6.5. (B) Size and purity of recombinant proteins based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (lane 1, R6.5 scFv; lane 2, hTM/R6.5) and (C) size exclusion high-performance liquid chromatography. hTM/R6.5 has a minor dimer form (indicated by arrow), accounting for ∼20% of absorbance at 280 nm. (D) hTM/R6.5 binds specifically to human ICAM-1–expressing CHO cells (CHO-hICAM), but not wild type (CHO wt). Flow cytometry was performed on each cell type incubated with different concentrations of fluorescently labeled fusion protein. For each reaction, MFI was calculated for 10 000 events. Each reaction was done twice, with mean ± standard deviation (SD) of the MFI shown. (E) hTM/R6.5 binds strongly to TNF-α–stimulated HUVEC but only minimally to nonstimulated ECs. Cell-based enzyme-linked immunosorbent assays were performed on live cells as previously described19 using anti–FLAG-HRP to probe for cell-bound fusion protein. Graph shows mean ± SD. (F) In vitro APC generation assay by cell-bound fusion protein, as described previously.19 TNF-α–stimulated HUVECs show reduced APC generation capacity due to loss of surface TM, although some residual function is seen in comparison with cells treated with an anti-TM blocking mAb. For testing the functional activity of cell-bound hTM/R6.5, TNF-α–stimulated cells were incubated for 30 min at 37°C with various concentrations of fusion protein and then washed to remove nonspecifically bound protein prior to incubation with human thrombin (1 nM) and PC (100 nM). At saturating concentrations of hTM/R6.5 (40-50 nM), the fusion protein more than compensates for the reduction in PC activation induced by TNF-α stimulation (*P < .01 vs TNF only). Graph shows mean ± SD, n = 3 for each condition. EC50, 50% effective concentration; mOD, 1/1000th of an optical density unit.