Figure 1.
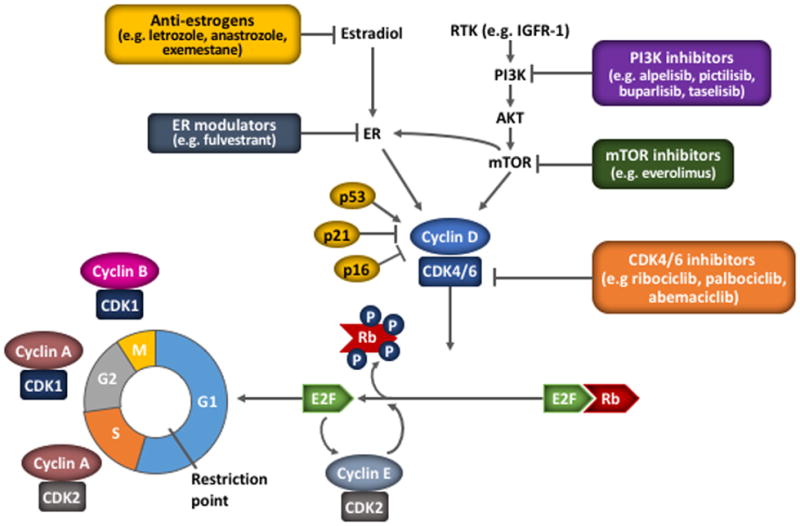
The role of cyclin–CDK complexes and the cyclin D–CDK4/6–p16–Rb pathway in the cell cycle
The cell cycle is regulated by cyclins and checkpoints, with different cyclins (and cyclin–CDK combinations) and checkpoints acting at phase transition of the cell cycle. At the G1–S cell cycle checkpoint, mutiple mitogenic pathways including ER and PI3K/AKT/mTOR promote the synthesis of cyclin D, which associates with and activates CDK4/6. Activated CDK4/6 phosphorylates the Rb protein to disrupt its sequestering interaction with E2F, releasing its transcription effect and allowing the expression of genes necessary for cell cycle progression, including cyclin E. Cyclin E associates with CDK2, which further phosphorylates Rb, resulting in progression of the cell cycle past the restriction point and irreversible S phase entry. Additional cyclin–CDK complexes act at further cell cycle checkpoints; cyclin A–CDK2 enables the S–G2 transition, and cyclin A–CDK1 and cyclin B–CDK1 facilitate the onset and progression of mitosis, respectively (82). Selective inhibitors of CDK4/6, such as ribociclib, act directly on the cyclin D–CDK4/6–p16–Rb pathway to block cell cycle progression. The pathway may also be impacted by inhibitors of other pathways acting upstream of CDK4/6, providing a rationale for dual inhibition.
CDK, cyclin-dependent kinase; ER, estrogen receptor; HER2, human epidermal growth factor receptor-2; IGF1R, insulin-like growth factor 1 receptor; MAPK, mitogen-activated protein kinase; RTK, receptor tyrosine kinase.
