Abstract
Purpose
Microperimetry is used as an endpoint in type 2 macular telangiectasia (mactel) trials. The change required for defining disease progression depends on measurement error. We determined the threshold of test–retest variability (TRV) of microperimetry in mactel.
Methods
A prospective study was done of 24 patients with stable mactel enrolled in a tertiary eye clinic. Each patient underwent three sessions of microperimetry separated by a median of 28 days. An identical testing protocol was used: 4-2 staircase algorithm at 37 loci radial grid covering central 6°. Microperimetry variables were compared across three visits. TRV was quantified by calculating the coefficients of repeatability (CRs) for mean and median foveal sensitivity and the number of loci with dense scotoma (DS) or normal sensitivity (NS). The 95% confidence intervals (CIs) for CRs were calculated.
Results
Mean and median foveal sensitivity increased from first to second testing sessions. Test duration, visual acuity, number of loci with DS, and fixation stability remained stable through the three test sessions. The intersession CRs for mean and median foveal sensitivity were 2.6 (95% CI, 1.8–3.3) and 2.4 (95% CI, 1.7–3.1) dB, respectively. CRs for the number of DS and NS loci were 5 and 12 loci. CR for both logBCEA63 and logBCEA95 was 1.0 (95% CI, 0.8–1.2).
Conclusions
The first microperimetry examination should be discarded due to learning effects. TRV in foveal sensitivity may be as high as 3.3 and 3.1 dB (∼0.3 log unit; 2× change) for its mean and median.
Translational Relevance
Our results have implications for the design of clinical trials in mactel.
Keywords: MAIA microperimetry, fixation stability, scotoma, retinal sensitivity, repeatability, measurement error
Introduction
Idiopathic type 2 macular telangiectasia (mactel), also referred to as juxtafoveolar retinal capillary telangiectasia, was first classified in detail in 1982 by Gass and Oyakawa1 with further elaboration on classification made in 1993 by Gass and Blodi.2 This is a bilateral disease characterized by progressive changes in the macular capillary network that follow a slowly progressive course, which begins temporal to the foveal center, in association with neurosensory atrophy and loss of luteal pigment, resulting in encroachment of paracentral scotoma into the foveal center.3 Visual acuity remains stable for many years in the early stages of the disease and, therefore, is not a suitable clinical endpoint in neuroprotective treatment trials.3 Microperimetry now has been adopted as a promising functional endpoint for monitoring visual decline in this disease.
Microperimetry is a modified visual field test that allows assessment of retinal differential light sensitivity at selected and known retinal loci and fixation characteristics without the need for stable and foveal fixation.4 This technique currently is used as an outcome measure in five type 2 mactel clinical trials registered with the United States National Institutes of Health clinical trial registry (clinicaltrials.gov–NCT 00685854; NCT 01327911; NCT 00685503; NCT 00504400; NCT 01949324).5 Quantification of the limits of test–retest variability (TRV) is essential in distinguishing disease progression from measurement error. In conventional perimetry, the limits of TRV has been shown to be different between healthy subjects and patients with reduced vision.6 Similarly, TRV of microperimetry has been investigated in healthy subjects, nonneovascular age-related macular degeneration (AMD), glaucoma, and other maculopathies.7–17
To our knowledge, TRV of microperimetry outcomes in type 2 macular telangiectasia has not been reported to date. Therefore, we conducted a prospective cohort study to determine the intersession TRV of fixation stability and retinal sensitivity measurements in patients with type 2 macular telangiectasia using the CenterVue MAcular Integrity Assessment (MAIA) microperimeter.
Methods
This study was approved by the Human Research Ethics Committee of the Sir Charles Gairdner Hospital (protocol, 2011-063), was registered with the Therapeutic Goods Administration of the Australian Government (CTN, 263/2012) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants before enrollment.
Participants
Patients were recruited between September 2011 and September 2013 through the local cohort of the International Macular Telangiectasia Registry18 and all subjects had been examined by a retinal specialist (IJC) for confirmation of the diagnosis, which was validated further by the Registry study reading center.18 Exclusion criteria included coexisting ocular conditions that potentially were unstable over the duration of the study (2 months), significant media opacity, or inability to give informed consent. These macular telangiectasia participants were recruited as part of a larger study involving two microperimeters–the CenterVue MAIA, as well as the Nidek MP-1. We report the results of MAIA microperimetry in subjects with type 2 mactel.
Ophthalmic Investigations
Participants were examined three times at three study visits separated by 1 month (visits 1 to 3). At each study visit, best-corrected visual acuity assessment using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart (Lighthouse International, New York, NY), MAIA microperimetry and spectral-domain optical coherence tomography (SD-OCT) imaging were performed. At the baseline visit (Test 1), if both eyes were eligible for the study, one eye was chosen at random to be the study eye using a random number generator (available in the public domain at www.random.org). If only one eye was eligible, that eye was chosen as the study eye.
Before any slit-lamp examination or imaging of the retina, the study eye was tested on MAIA and MP-1 microperimeters consecutively. The order of testing was assigned by a list generated by the online random number generator before commencement of the study for all study visits. Microperimetry testing is detailed in the section below. Pupil dilation was not performed.
Following completion of microperimetry tests, all participants underwent multimodal imaging using the Spectralis HRA+OCT device (Heidelberg Engineering, Heidelberg, Germany). Infrared reflectance fundus image, macular cube volume scans (covering 30° × 25° area at scan separation of 120 μm), and optic disc nerve fiber layer circular scans were acquired. All scans were examined to ensure no disease progression had occurred during the three study visits.
Microperimetry Testing Protocol
Microperimetry was performed using the CenterVue MAIA (CenterVue, Padova, Italy). Participants were provided with identical instructions on how to perform the test at each study visit. There was no practice test before the first session. However, participants were given the opportunity to familiarize themselves with the response trigger. Testing was conducted in a dedicated quiet psychophysics dark room, and the lights were turned off after the nonstudy eye was patched for at least 5 minutes before commencement of testing. The patient was positioned comfortably at the device with chin on the chin rest and finger ready to press the response trigger.
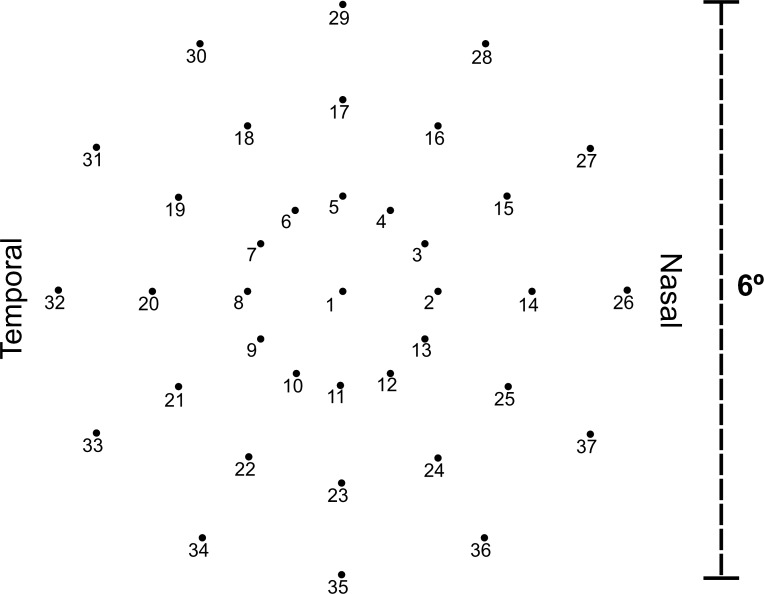
The fixation target was a 2° diameter red ring broken into four segments. The dim white background was set at a luminance level of 1.27 cd/m2 and the maximum stimulus intensity achieved by the laser was 318.31 cd/m2 producing a dynamic range 317.04 cd/m2. The differential stimulus luminance can be attenuated from 0 dB (at maximum differential luminance of 317.04 cd/m2) to 36 dB (at minimum differential luminance 0.08 cd/m2) in steps of 1 dB. Stimulus size was Goldmann III, duration was 200 ms, and testing protocol was a 4-2 threshold strategy. The standard 6° grid was used, which consisted of 37 test loci arranged in a radial pattern (Fig. 1).
Figure 1.
The 37 test loci grid pattern covers the central 6° region of the retina with 1° separation between each of the 3 rings. In MAIA, light stimuli created by a white LED light are projected directly onto the retinal surface at specific locations. Subsequent measurements re-examines the same anatomical locations by using the follow-up retest protocol.
The MAIA microperimeter has a built-in whole-fundus registration software function that allows follow-up tests to be registered automatically to the baseline test; thus, enabling accurate reassessment of retinal sensitivity at the same test loci examined during the baseline test.
We reported the microperimetry outcome variables that have been defined previously, including mean foveal sensitivity7 ( ; average of retinal sensitivity across all 37 loci), median foveal sensitivity17 (
; average of retinal sensitivity across all 37 loci), median foveal sensitivity17 (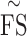 ; median of retinal sensitivity across all 37 loci), number of dense scotoma17 (DS; number of loci at which there was no response to the brightest stimulus), and number of loci with normal sensitivity (NS; number of loci with retinal sensitivity of 25 dB or greater). For pointwise sensitivity analysis, we transposed all left eye data onto a right eye grid, and analyzed TRV of corresponding test loci across the whole cohort. A score of −1 dB was assigned if a participant had no response to the brightest stimulus (as opposed to a score of 0 dB if the participant did respond to it); therefore, the maximum range was 37 dB.
; median of retinal sensitivity across all 37 loci), number of dense scotoma17 (DS; number of loci at which there was no response to the brightest stimulus), and number of loci with normal sensitivity (NS; number of loci with retinal sensitivity of 25 dB or greater). For pointwise sensitivity analysis, we transposed all left eye data onto a right eye grid, and analyzed TRV of corresponding test loci across the whole cohort. A score of −1 dB was assigned if a participant had no response to the brightest stimulus (as opposed to a score of 0 dB if the participant did respond to it); therefore, the maximum range was 37 dB.
The MAIA microperimeter also quantifies fixation stability. The proportion of fixation points that fall within 1° and 2° radius are termed P1 and P2, respectively. It then classifies a subject's fixation stability into stable (P1 > 75%), relatively unstable (P1 < 75% and P2 > 75%), or unstable (P2 < 75%). The MAIA also measures the bivariate contour ellipse area (BCEA) for 63% and 95% of all measured fixation points. These variables were normalized by a logarithm (of base 10) transformation before statistical analysis and comparison with previous studies.19 Logarithm of BCEA (logBCEA) was calculated for the 63% and 95% values.
Statistical Analysis
The Shapiro-Wilk test was used to determine if data were distributed normally. Nonparametric statistical analyses were used for data that did not conform to a normal distribution. Median and interquartile range (IQR) was reported for the study variables.
The nonparametric Friedman test was used to determine if changes had taken place in test duration, visual acuity, fixation stability, mean or median foveal sensitivity, and number of DS and NS across the three visits. The Wilcoxon signed ranks test was used to compare the intersession intervals among the three test sessions.
Bland-Altman plots20 were used first to assess test–retest characteristics across the range of magnitude of outcome measures, and the presence of systematic changes were assessed by calculating the rank correlation coefficient (Kendall tau-b). We quantified the intersession coefficients of repeatability (CR) for each outcome measure based on the formula recommended by Bland and Altman21:
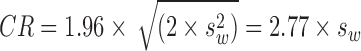 |
where  is mean within-subject variance, which was derived from the sum of squares about the subject mean divided by the degrees of freedom. The 95% confidence interval (CI) for the CR can be calculated based on the assumption that the square root of a χ2 variable,
is mean within-subject variance, which was derived from the sum of squares about the subject mean divided by the degrees of freedom. The 95% confidence interval (CI) for the CR can be calculated based on the assumption that the square root of a χ2 variable,  , has an approximately normal distribution with the variance being approximately ½.22 Standard error (SE) of
, has an approximately normal distribution with the variance being approximately ½.22 Standard error (SE) of 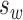 , was estimated by the following formula based on the number of subjects (n) and number of repetitions (m):
, was estimated by the following formula based on the number of subjects (n) and number of repetitions (m):
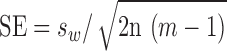 |
 |
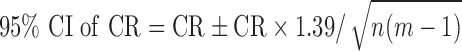 |
For variables that had logarithm transformation, back transformation (through antilog) of 1.96 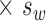 was calculated to derive a ratio that determines test–retest variability relative to any given measurement.
was calculated to derive a ratio that determines test–retest variability relative to any given measurement.
Significance was set at a P value of <0.05. All statistical analyses were performed on the commercially available Statistical Package for Social Sciences (SPSS, software version 21, IBM Corporation, Armonk, NY). A summary of all symbols and abbreviations is found in Supplementary Table S1.
Results
A total of 24 participants (11 female and 13 male subjects; median age [range], 63 [44–81] years; eight right and 16 left eyes) were recruited into the study. Median (IQR) intervals between Tests 1 and 2, and Tests 2 and 3, were 28 (28–31) and 28 (28–33) days, respectively (Wilcoxon signed rank test, P = 0.81). All enrolled subjects completed three study visits. There was no significant difference in the intervals between the study visits (paired t-test, P = 0.50). There was no significant difference between median durations (Friedman test, χ2[2] = 0.158, P = 0.45). The median (IQR) duration for each microperimetry examination was 5 minutes 18 seconds (5 minutes 6 seconds–5 minutes 42 seconds), 5 minutes 10 seconds (5 minutes 0 seconds–5 minutes 22 seconds), and 5 minutes 11 seconds (4 minutes 56 seconds–5 minutes 34 seconds) for the three study visits, respectively. Fixation stability was assessed as stable, relatively unstable, and unstable in 18, 5, and 1 subject, respectively, at baseline test. All microperimetry tests met a minimum reliability index standard of 75% (mean 96.4%, range 75–100%).
Disease Stability
Quantitative and qualitative review of SD-OCT macular cube scans showed no change in retinal volume or thickness beyond test–retest variability and no disease progression in any of the study eyes. Median (IQR) best-corrected visual acuities remained stable through the three study visits, respectively (Friedman test, χ2[2] = 0.437, P = 0.80, Table 1).
Table 1.
Outcome Measures Across Three Testing Visits
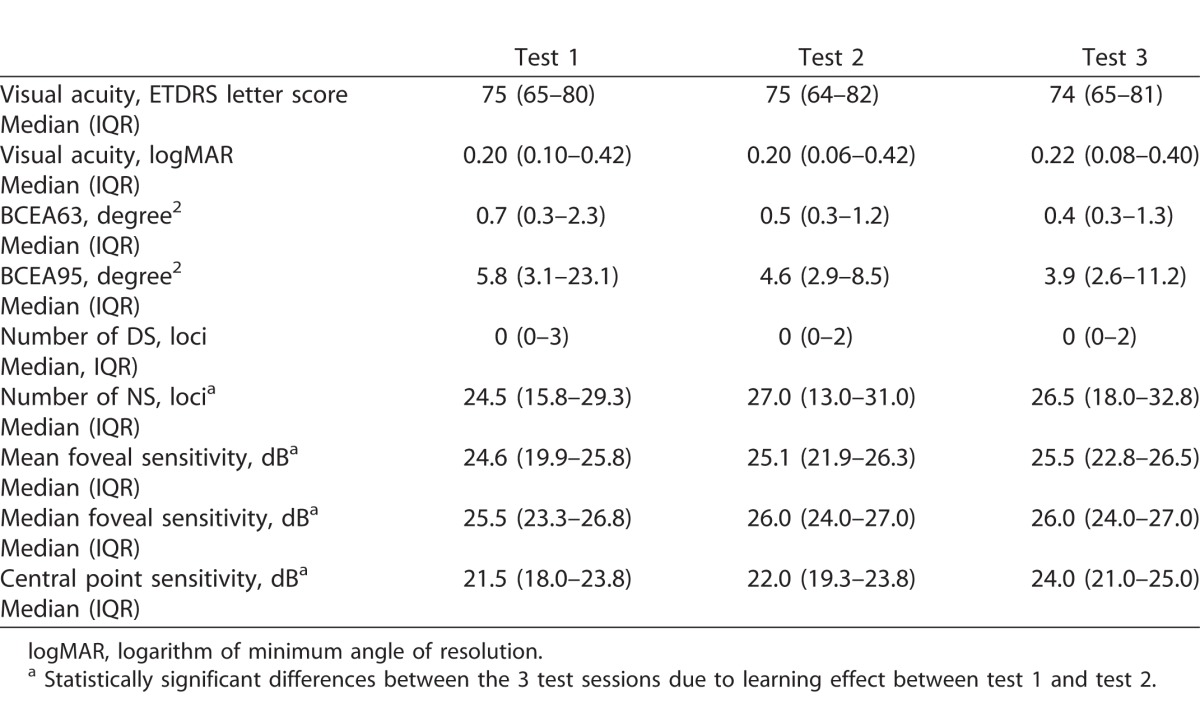
There was no significant change in median (IQR) BCEA that encompassed 63% or 95% of fixation loci (Friedman test, P = 0.92 and 0.87, respectively). Similarly, logBCEA63 and logBCEA95 also did not change across three study visits (Friedman test, P = 0.92 and 0.87), respectively. The number of loci with DS also remained stable (Friedman test, P = 0.15). In contrast, there was a trend for increasing number of loci with NS over three test sessions (Friedman test, P = 0.01).
The medians (IQRs) of 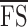 were 24.6 (19.9–25.8), 25.1 (21.9–26.3), and 25.5 (22.8–26.5) dB over the three study visits, respectively. Friedman test revealed statistically significant differences among the three visits (χ2[2] = 15.290, P < 0.001). Post hoc analysis with Wilcoxon signed rank tests was conducted with a significance level set at P < 0.05. Significant differences were found between Tests 1 and 2 (P = 0.003) and Tests 1 and 3 (P = 0.002), but not between Tests 2 and 3 (P = 0.095). A similar trend also was found for the median of
were 24.6 (19.9–25.8), 25.1 (21.9–26.3), and 25.5 (22.8–26.5) dB over the three study visits, respectively. Friedman test revealed statistically significant differences among the three visits (χ2[2] = 15.290, P < 0.001). Post hoc analysis with Wilcoxon signed rank tests was conducted with a significance level set at P < 0.05. Significant differences were found between Tests 1 and 2 (P = 0.003) and Tests 1 and 3 (P = 0.002), but not between Tests 2 and 3 (P = 0.095). A similar trend also was found for the median of 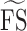 where Test 1 (25.5 dB) was significantly worse than Tests 2 (26.0 dB) and 3 (26.0 dB). Therefore, we excluded the results of Test 1 and included only data from Tests 2 and 3 for analysis of measurement error. Based on a sample size of 24 with 2 test trials, the 95% CI for CR is 28% above and below the estimated value.
where Test 1 (25.5 dB) was significantly worse than Tests 2 (26.0 dB) and 3 (26.0 dB). Therefore, we excluded the results of Test 1 and included only data from Tests 2 and 3 for analysis of measurement error. Based on a sample size of 24 with 2 test trials, the 95% CI for CR is 28% above and below the estimated value.
To analyze the impact of order of microperimetry testing on the change in mean foveal sensitivity between the first and second examinations, we divided the cohort into three groups: (1) those who underwent MAIA before MP-1 on both examinations (n = 5), (2) those who underwent MP-1 before MAIA on both examinations (n = 6), and (3) those underwent MP-1 before MAIA on the first examination and then MAIA before MP-1 on the second examination (N = 11). There was minimal order effect on sensitivity change between study visits except for the second group, which showed a statistically significant increase in mean foveal sensitivity between the first and the second sessions (P = 0.02).
Intersession Test–Retest Variability
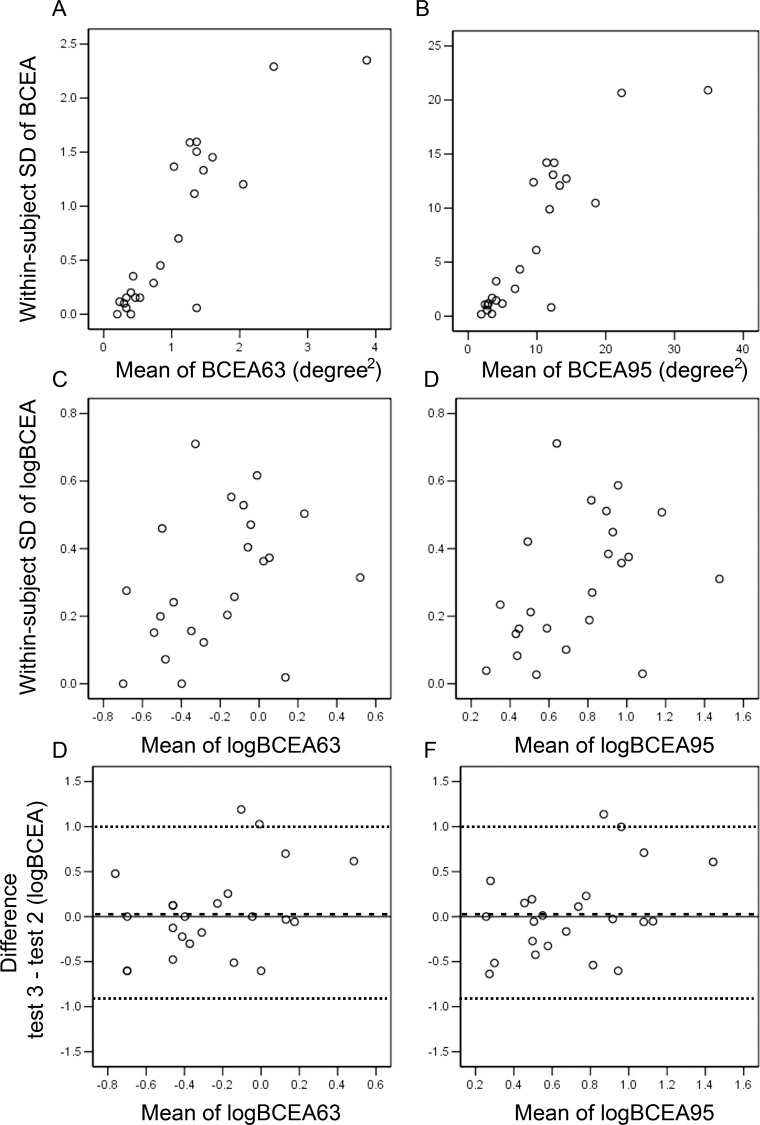
Bland-Altman analysis showed that variability in BCEA increased with its magnitude (Kendall's tau = 0.65 and 0.69 for BCEA63 and BCEA95, respectively, P < 0.001, Fig. 2). After logarithmic transformation, the relationship was less apparent on Bland-Altman analysis (Kendall's tau = 0.29 and 0.30, P = 0.06 and 0.04 for logBCEA63 and logBCEA95, respectively). Based on a 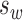 of 0.36 and a standard error of 20% in logBCEA, a change in BCEA by 5× (95% CI, 3.7 to 7.2) can be considered to exceed test–retest variability. The CRs for logBCEA are shown in Table 2.
of 0.36 and a standard error of 20% in logBCEA, a change in BCEA by 5× (95% CI, 3.7 to 7.2) can be considered to exceed test–retest variability. The CRs for logBCEA are shown in Table 2.
Figure 2.
A Bland-Altman plot of within-subject standard deviation (SD) against mean shows increasing variability of the BCEA with magnitude for 63% (A) and 95% (B) of fixation loci. After logarithm (base 10) transformation, variability became independent of mean for logBCEA 63% (C) and logBCEA 95% (D). Bland-Altman plots of difference against mean show similar measurement error between logBCEA 63% (E) and logBCEA 95% (F). The mean difference (test 3–test 2) is marked as dashed line (- - - ) and the 95% limits of agreement (2 × SD above and below mean) are marked as dotted lines (. . . ).
Table 2.
Coefficients of Repeatability for Outcome Variables
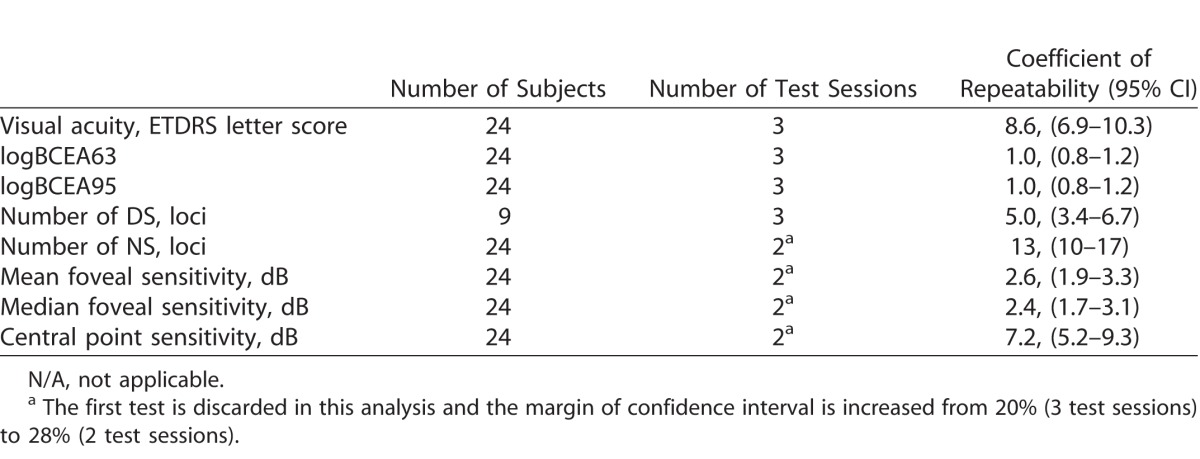
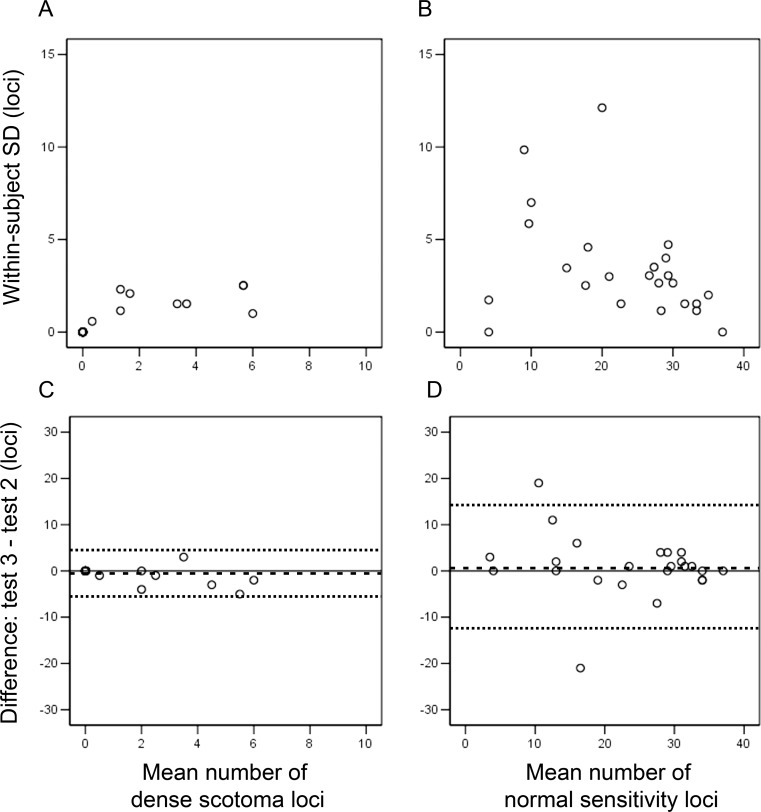
In nine of 24 subjects, a DS was identified at one of the three microperimetry tests. The estimated CR (95% CI, at ±33%) for number of DS was 5.0 (3.4–6.7) loci. For the number of NS loci, the estimated CR (95% CI at ±20%) was 12.3 (9.9–14.8, Fig. 3). Figure 4 shows three cases where there was a significant variation in the number of DS and NS loci across three visits.
Figure 3.
A Bland-Altman plot of within-subject SD against mean shows variability of the number of loci with dense scotoma (A) and normal sensitivity (B) did not increase with magnitude. Bland-Altman plot of difference against mean show dense scotoma loci mapping (C) is slightly more reliable than normal sensitivity loci mapping (D). The mean difference (test 3–test 2) is marked as dashed line (- - -) and the 95% limits of agreement (2 × SD above and below mean) are marked as dotted lines (. . .). Floor effects were removed during calculation of limits of agreement.
Figure 4.
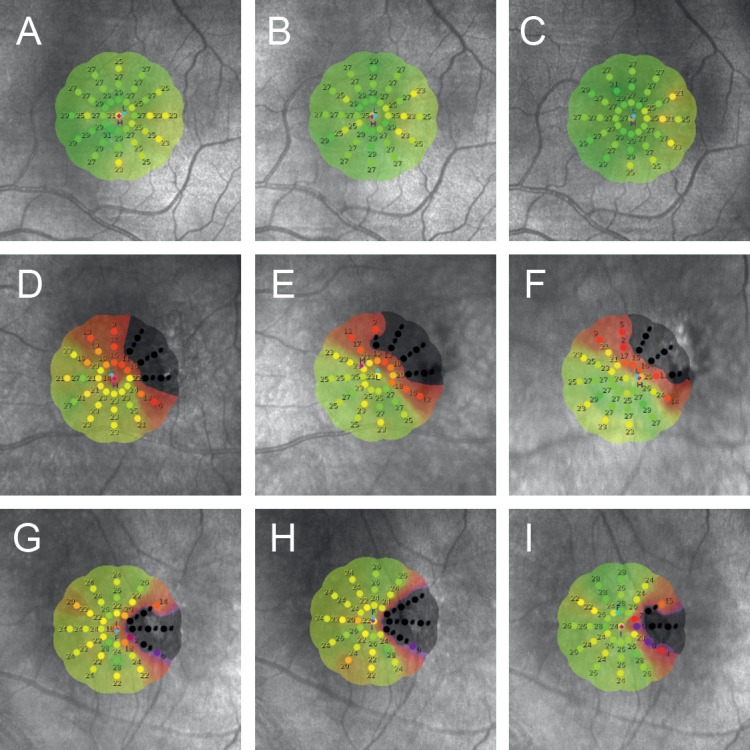
Three examples of interpolated sensitivity maps for three visits illustrating a subject with no scotoma and stable fixation (A–C), a subject with scotoma but stable fixation (D–F), and a subject with scotoma and relatively unstable fixation (G–I). They illustrate the variability in dense scotoma and normal sensitivity loci mapping.
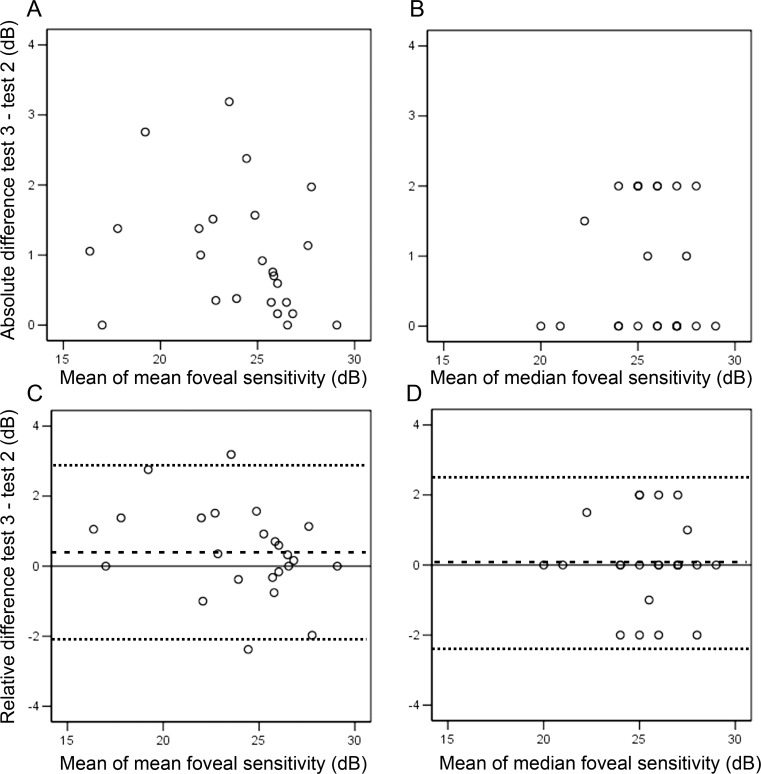
CRs for 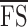 and
and 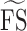 were 2.6 (95% CI, 1.8–3.3) and 2.4 (95% CI, 1.7–3.1) dB, respectively. Bland-Altman plots for each outcome measure are shown in Figure 5. A sensitivity analysis was performed by dividing our cohort into those without (n = 16) and with (n = 8) dense scotoma. The CRs for
were 2.6 (95% CI, 1.8–3.3) and 2.4 (95% CI, 1.7–3.1) dB, respectively. Bland-Altman plots for each outcome measure are shown in Figure 5. A sensitivity analysis was performed by dividing our cohort into those without (n = 16) and with (n = 8) dense scotoma. The CRs for 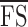 were 2.5 and 3.0 dB, respectively. The CRs for
were 2.5 and 3.0 dB, respectively. The CRs for 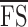 in those with stable (n = 18) and relatively unstable or unstable fixation (n = 6) were 2.3 and 3.4 dB, respectively.
in those with stable (n = 18) and relatively unstable or unstable fixation (n = 6) were 2.3 and 3.4 dB, respectively.
Figure 5.
Bland-Altman plots of absolute difference against mean show no change in variability of mean (A) and median (B) foveal sensitivity across a range of measurement magnitude. Bland-Altman plots of difference against mean show similar extent of measurement error between mean (C) and median (D) foveal sensitivity. The mean difference (test 3–test 2) is marked as dashed line (- - -) and the 95% limits of agreement (2 × SD above and below mean) are marked as dotted lines (. . .).
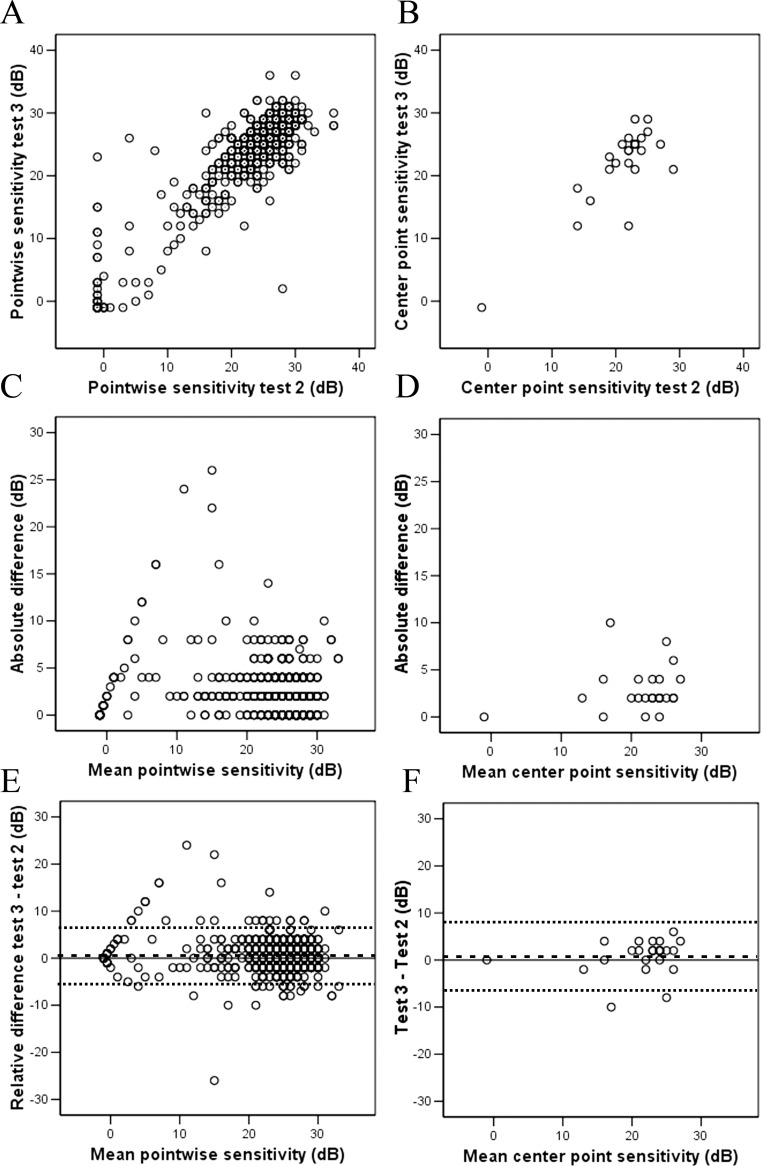
Pointwise analysis was performed on 888 test loci (37 loci × 24 subjects) between Tests 2 and 3. The mean (SD) relative difference (Test 3–Test 2) in pointwise sensitivity was 0.42 (3.3) dB. Median (range) absolute difference was 2 (0–26) dB. A total 850 (95.7%) pairs of measurements had an absolute difference in pointwise sensitivity of 6 dB or less; 30.4% of pairs of measurements had identical pointwise sensitivity. A plot of pointwise sensitivity values at Test 3 versus Test 2 showed an increase in variability as baseline sensitivity decreased. This also is highlighted by Bland-Altman plot of differences against mean (Fig. 6). A sensitivity analysis was performed using the center test loci (Fig. 6). The CR (95% CI) for this location was 7.2 (5.2–9.3) dB.
Figure 6.
Scatter graphs show some consistency in pointwise sensitivity measurements between Tests 2 and 3 in pooled analysis (A, all loci across all patients = 888 pairs of measurements) and foveal center locus (B, one locus across all patients = 24 pairs of measurements). Variability is most marked in retinal loci with a mean retinal sensitivity of less than 20 dB in pooled analysis (C) but this was not obvious at foveal center locus (D) because of foveal sparing lesions in macular telangiectasia. Bland-Altman plots of differences against mean show that measurement error is driven by certain retinal loci with poorer mean sensitivity in pooled analysis (E) but not at foveal center locus (F). The mean difference (Test 3–Test 2) is marked as dashed line (- - -) and the 95% limits of agreement (2 × SD above and below mean) are marked as dotted lines (. . .). Floor effects were removed during calculation of limits of agreement.
Discussion
We investigated the TRV over three test sessions with the objective of understanding the degree of disease-specific measurement error of foveal function mapping in type 2 macular telangiectasia. We found a significant learning effect in overall retinal sensitivity between first and second test sessions and, therefore, we used only the data from the second and the third tests for the analysis of TRV. We showed that intersession measurement error can be as large as 3.3 and 3.1 dB (∼0.3 log unit; 2× change in differential luminance) for mean and median foveal sensitivities. Pointwise sensitivity may change up to 6 dB in 95% of test loci and measurement error can be as large as 9 dB when the central point is considered in isolation. We also found a CR for fixation stability of up to 1.0 logBCEA units (10× change in BCEA).
Our observation of a learning effect is consistent with the findings of Wu et al.,11 who described a learning effect in their study examining intrasession TRV with MAIA microperimetry in a cohort of patients with AMD. However, Molina-Martin et al.23 reported no intersession learning effect in healthy subjects, a finding consistent with numerous other studies using other microperimetry devices.7,24–27 In three of these studies, however, a truncated training session was used before commencement of the first microperimetry test and no learning effect was noted.7,24,27 In our cohort, two subjects had prior experience of microperimetry and when analyzed separately these two subjects did not show a learning effect. We also found significant learning effect when MAIA microperimetry was performed after MP-1 at Tests 1 and 2. This unexpected finding could be explained by a larger learning effect in between the two consecutive tests in Test 2 then Test 1. However, our sample size was too small to test this hypothesis. Although there is inconsistency in the current published literature, our findings compel us to recommend discarding microperimetry data from the first session and using the results from the second session as a baseline from which to determine progression. It remains to be determined whether an abbreviated mock training session immediately before the baseline microperimetry is as effective as a full microperimetry test identical to the baseline in eliminating learning effect.
The intersession repeatability for the mean macular sensitivity with MAIA microperimetry has been reported in normal subjects.23 Since CR was not calculated, a direct comparison is not possible. Intrasession CR for the mean macular sensitivity has been reported in 30 nonneovascular AMD (1.08 [95% CI, 0.83–1.33] dB) and 14 normal subjects (1.10 [95% CI, 0.71–1.50] dB).10 An additional AMD cohort of 71 subjects (visual acuity range of 46–63 letters) also was examined in the same study and had CR for the mean macular sensitivity of 1.56 (95% CI, 1.33–1.80) dB. The CR for the mean foveal sensitivity in our study was significantly larger than that previously reported by Wu et al.,10–12 for healthy subjects, and both AMD cohorts, with no overlap of the 95% CI. There are a number of possible reasons for this discrepancy. Firstly, our study had an intersession rather than an intrasession study design, as in the study by Wu et al.11 While differences between inter- versus intrasession variability in microperimetry have not been examined, a recent study examined such differences in 17 patients with optic neuritis, and 10 healthy controls using conventional perimetry. They found reduced variability in intersession compared to intrasession TRV for healthy controls, but the reverse for the optic neuritis group.28 Secondly, the cohorts from the study by Wu et al.11 consisted of either healthy controls, or nonneovascular AMD patients with a single druse greater than or equal to 125 μm or multiple drusen greater than or equal to 63 μm or noncentral geographic atrophy. The study participants in the healthy controls and nonneovascular AMD may have better visual acuity and fixation than our cohort. Unfortunately, a comparison cannot be made because this information was not reported in that study. A quarter (six of 24) of our study cohort had relatively unstable or unstable fixation quality and this may contribute to the higher TRV. The increasing variability in the mean foveal sensitivity associated with increasing levels of visual deficit demonstrated in our study requires further investigation to identify whether it will be appropriate to apply specific thresholds at each level of functional deficit.
We have reported previously CR for median macular sensitivity in a cohort of 16 subjects with a range of conditions.17 The reason for using median as opposed to mean as the summary statistics for the overall retinal sensitivity measure is that the distribution of sensitivity values cannot be assumed to be Gaussian. This is particularly relevant in type 2 macular telangiectasia where test loci with normal sensitivity may surround scotomatous test loci within the temporal perifoveal neurodegeneration. In our previous study, we reported CR for the median macular sensitivity as 3.44 (95% CI, 2.31–4.56) dB, which was comparable to the CR for the median foveal sensitivity in our current study. Pointwise sensitivity measurement also was similar between the two test sessions, although only 30% had identical values as opposed to 47% to 51% as shown in our previous studies using a different microperimeter.8,17 Almost 96% of loci had a change of pointwise sensitivity of up to 6 dB (∼0.6 log unit; 4× change in differential luminance) and this is comparable to our previous finding of 97% using the MP-1 device.8
In addition to retinal sensitivity measurements, the number of test loci with dense scotoma (<0 dB) or normal sensitivity (≥24 dB) also can be used to quantify disease progression. Fixation stability also can deteriorate as the scotoma encroaches into the fovea. Therefore, change in fixation stability also can be used for detecting disease progression. We previously reported the CR for the number of dense scotoma loci and normal sensitivity loci as 3 and 10 loci,17 which are slightly lower than 5 and 13 loci in type 2 macular telangiectasia. Taking into consideration the small sample size, we would consider an increase of seven dense scotoma loci or a reduction of 15 normal sensitivity loci as the cutoff margin for defining disease progression. Our previous study of repeatability in fixation stability measurement using the MP-1 microperimeter showed that CR for logBCEA was 0.61 (95% CI, 0.49–0.73). In our cohort, the CR for fixation stability measure was 1 logBCEA unit; 10× change in BCEA. It is difficult to compare these values because the fixation target used by MAIA device is different from MP-1 device and the BCEA values from MAIA is in degree2 rather than minute arc2.19 Nevertheless, a fivefold test–retest variability in BCEA values derived from MAIA suggests that this may not be a suitable variable for measuring disease progression.
Our study has several limitations. First, our sample size was small and we only performed three microperimetry examinations. We limited the number of test sessions because of the rarity of the disease and the likelihood of reduced participation rate from this patient cohort if further microperimetry testing was required. The use of only two of the three test session data to calculate within-subject standard deviation increased the confidence interval margin of the CR from 20% to 28%. We had to discard some of the variables measured at the first study visit because of learning effect. Although increasing the number of microperimetry test sessions would allow us to examine long-term learning effect and reduce standard error, this also would reduce study compliance and introduce variability from disease progression. Second, we only examined patients with relatively good visual acuity and stable fixation. Therefore, the generalizability of our results to patients with end-stage macular telangiectasia remains to be determined because we did not include patients with poor acuity or unstable fixation. Future studies should include patients with more advanced disease (poorer fixation and more numerous dense scotoma) as well as subclinical diseases (better fixation and higher median and mean retinal sensitivity values). Third, we used a relatively small radial testing grid pattern that only covered the central 6° of visual field (the foveal region). Therefore, our results and the threshold values we obtained may not be readily comparable to those produced using other testing grids, such as the much larger Cartesian testing grid that covers parafoveal and perifoveal regions as is used in current neuroprotective treatment trials of type 2 macular telangiectasia. Fourth, we tested only one eye in each subject, whereas both eyes are tested in a clinical trials setting. The relative effect of intrasession, interocular variability over intersession variability could not be investigated in this study design. However, we found differences in learning effect when the order of microperimetry device used was altered within each session. Future studies with larger sample size and bilateral testing are required to answer these questions.
In conclusion, we recommended against the use of results from the first session of microperimetry as the baseline in clinical trials of macular telangiectasia because of the significant learning effect in retinal sensitivity between the first and the second tests. Future studies are required to investigate whether an abbreviated mock training is as effective as a full microperimetry test (same as the baseline) in reducing learning effect. A decline of 3.3 and 3.1 dB in the mean and median foveal sensitivities can be considered as disease progression. An increase in the number of loci with dense scotoma by up to 5 or a reduction in the number of loci with normal sensitivity by up to 13 can occur due to measurement error. We caution the use of pointwise sensitivity decline or BCEA enlargement to determine disease progression.
Supplementary Material
Acknowledgments
Supported by Sylvia and Charles Viertel Clinical Investigatorship Grant (FKC), Ophthalmic Research Institute of Australia Research Grant (FKC, ENW), Lions Eye Institute (FKC), Retina Australia Research Grant (FKC), and the NH&MRC Early Career Fellowship (APP1054712, FKC). The Lowy Medical Research Foundation supports the Mactel Patient Registry.
Disclosures: E.N. Wong, None; J.D.A. De Soyza, None; D.A. Mackey, None; I.J. Constable, None; F.K. Chen, None
References
- 1. Gass JDM, Oyakawa RT. . Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol. 1982; 100: 769– 780. [DOI] [PubMed] [Google Scholar]
- 2. Gass JDM, Blodi BA. . Idiopathic juxtafoveolar retinal telangiectasis: update of classification and follow-up study. Ophthalmology. 1993; 100: 1536– 1546. [PubMed] [Google Scholar]
- 3. Finger RP, Charbel Issa P, Fimmers R, Holtz FG, Rubin GS, Scholl HPN. . Reading performance is reduced by parafoveal scotoma in patients with macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2009; 50: 1366– 1370. [DOI] [PubMed] [Google Scholar]
- 4. Charbel Issa P, Helb H-M, Holz FG, Scholl HPN, MacTel Study Group . Correlation of macular function with retinal thickness in nonproliferative type 2 idiopathic macular telangiectasia. Am J Ophthalmol. 2008; 145: 169– 175. [DOI] [PubMed] [Google Scholar]
- 5. U.S. National Institutes of Health. Clinical Trials. Available at: http://clinicaltrials.gov. Accessed November 2, 2016.
- 6. Chauhan BC, Johnson CA. . Test-retest variability of frequency-doubling perimetry and conventional perimetry in glaucoma patients and normal subjects. Invest Ophthalmol Vis Sci. 1999; 40: 648– 656. [PubMed] [Google Scholar]
- 7. Anastasakis A, Mcanany JJ, Fishman GA, Seiple WH. . Clinical value, normative retinal sensitivity values, and intrasession repeatability using a combined spectral domain optical coherence tomography/scanning laser ophthalmoscope microperimeter. Eye (Lond). 2011; 25: 245– 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen FK, Patel PJ, Xing W, Bunce C, Egan C, Tufail AT,et al. . Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009; 50: 3464– 3472. [DOI] [PubMed] [Google Scholar]
- 9. Cideciyan AV, Swider M, Aleman TS,et al. . Macular function in macular degenerations: Repeatability of microperimetry as a potential outcome measure for ABCA4-associated retinopathy trials. Invest Ophthalmol Vis Sci. 2012; 53: 841– 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu Z, Ayton LN, Guymer RH, Luu CD. . Intrasession test-retest variability of microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54: 7378– 7385. [DOI] [PubMed] [Google Scholar]
- 11. Wu Z, Jung CJ, Ayton LN, Luu CD, Guymer RH. . Test-retest repeatability of microperimetry at the border of deep scotomas. Invest Ophthalmol Vis Sci. 2015; 56: 606– 2611. [DOI] [PubMed] [Google Scholar]
- 12. Wu Z, McKendrick AM, Hadoux X,et al. . Test-retest variability of fundus-tracked perimetry at the peripapillary region in open angle glaucoma. Invest Ophthalmol Vis Sci. 2016; 57: 3619– 3625. [DOI] [PubMed] [Google Scholar]
- 13. Jeffrey BG, Cukras CA, Vitale S, Turriff A, Bowles K, Sieving PA. . Test–retest intervisit variability of functional and structural parameters in X-linked retinoschisis. Trans Vis Sci Tech. 2014; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu H, Bittencourt MG, Wang J,et al. . Retinal sensitivity is a valuable complementary measurement to visual acuity — a microperimetry study in patients with maculopathies. Graefes Arch Clin Exp Ophthalmol. 2015; 253: 2137– 2142. [DOI] [PubMed] [Google Scholar]
- 15. Bedell HE, Pratt JD, Krishnan A,et al. . Repeatability of Nidek MP-1 Fixation measurements in patients with bilateral central field loss. Invest Ophthalmol Vis Sci. 2015; 56: 2624– 2630. [DOI] [PubMed] [Google Scholar]
- 16. Ismail SA, Sharanjeet-Kaur, Mutalib HA, Ngah NF. . Macular retinal sensitivity using MP-1 in healthy Malaysian subjects of different ages. J Optom. 2015; 8: 266– 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong EN, Mackey DA, Morgan WH, Chen FK. . Intersession test-retest variability of conventional and novel parameters using the MP-1 microperimeter. Clin Ophthalmol. 2015; 10: 29– 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Lowy Medical Research Institute. Mactel Registry. Available at: http://www.lmri.net/mactel-registry/. Accessed November 2, 2016.
- 19. Chen FK, Patel PJ, Xing W, Crossland MD, Bunce C, Rubin GS,et al. . Intrasession repeatability of fixation stability assessment with the Nidek MP-1. Optom Vis Sci. 2011; 88: 742– 750. [DOI] [PubMed] [Google Scholar]
- 20. Bland JM, Altman DG. . Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1: 307– 310. [PubMed] [Google Scholar]
- 21. Bland JM, Altman DG. . Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007; 17: 571– 582. [DOI] [PubMed] [Google Scholar]
- 22. Bland JM, . Department of Health Sciences, University of York: Available at: http://www-users.york.ac.uk/∼mb55/meas/seofsw.htm. Accessed November 2, 2016. [Google Scholar]
- 23. Molina-Martín A, Piñero DP, Pérez-Cambrodí RJ. . Reliability and intersession agreement of microperimetric and fixation measurements obtained with a new microperimeter in normal eyes. Curr Eye Res. 2016; 41: 400– 409. [DOI] [PubMed] [Google Scholar]
- 24. Chen FK, Patel PJ, Webster AR, Coffey PJ, Tufail A, Da Cruz L. . Nidek MP1 is able to detect subtle decline in function in inherited and age-related atrophic macular disease with stable visual acuity. Retina. 2011; 31: 371– 379. [DOI] [PubMed] [Google Scholar]
- 25. Acton JH, Bartlett NS, Greenstein VC. . Comparing the Nidek MP-1 and Humphrey Field Analyzer in normal subjects. Optom Vis Sci. 2011; 88: 1288– 1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Midena E, Vujosevic S, Cavarzeran F, Group MS. . Normal values for fundus perimetry with the microperimeter MP1. Ophthalmology. 2010; 117: 1571– 1571. [DOI] [PubMed] [Google Scholar]
- 27. Chandramohan A, Stinett SS, Petrowski JT,et al. . Visual function measures in early and intermediate age-related macular degeneration. Retina. 2016; 36: 1021– 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wall M, Johnson CA, Kutzko KE, Nguyen R, Brito C, Keltner JL. . Long- and short-term variability of automated perimetry results in patients with optic neuritis and healthy subjects. Arch Ophthalmol. 1998; 116: 53– 61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.