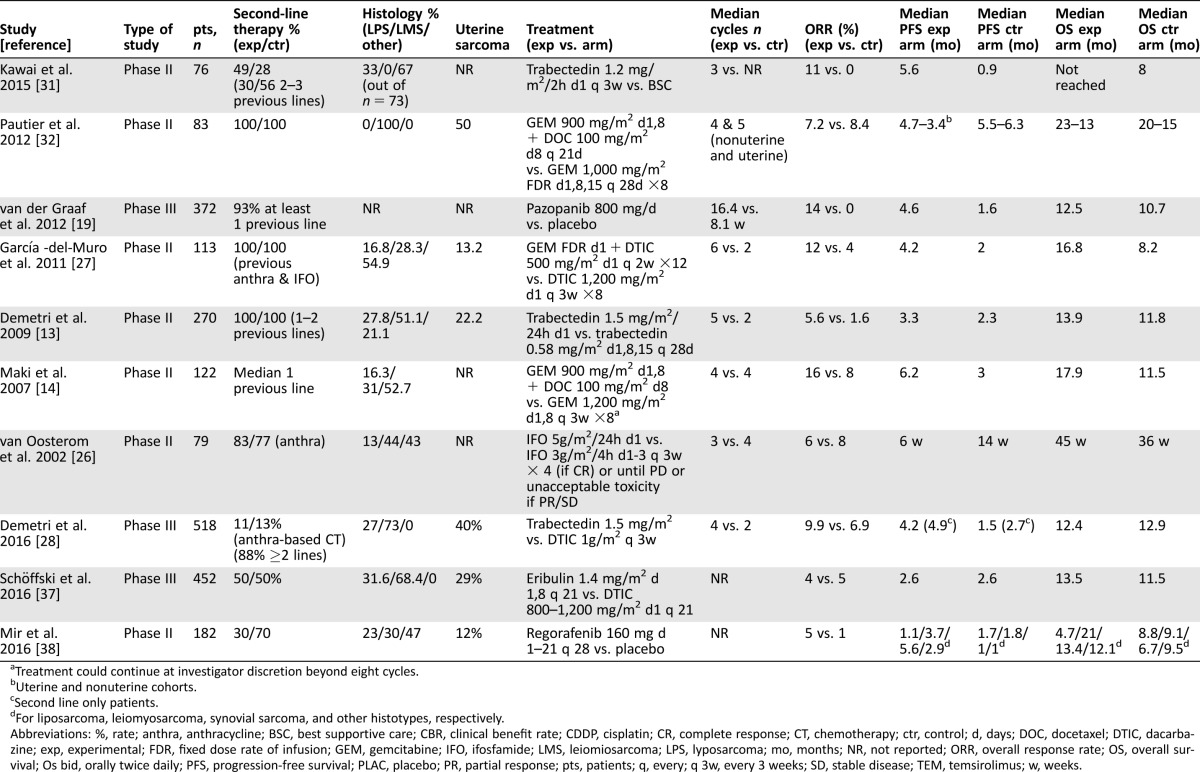
Table 1. Characteristics of the included studies.
Treatment could continue at investigator discretion beyond eight cycles.
Uterine and nonuterine cohorts.
Second line only patients.
For liposarcoma, leiomyosarcoma, synovial sarcoma, and other histotypes, respectively.
Abbreviations: %, rate; anthra, anthracycline; BSC, best supportive care; CBR, clinical benefit rate; CDDP, cisplatin; CR, complete response; CT, chemotherapy; ctr, control; d, days; DOC, docetaxel; DTIC, dacarbazine; exp, experimental; FDR, fixed dose rate of infusion; GEM, gemcitabine; IFO, ifosfamide; LMS, leiomiosarcoma; LPS, lyposarcoma; mo, months; NR, not reported; ORR, overall response rate; OS, overall survival; Os bid, orally twice daily; PFS, progression‐free survival; PLAC, placebo; PR, partial response; pts, patients; q, every; q 3w, every 3 weeks; SD, stable disease; TEM, temsirolimus; w, weeks.