Abstract
Background
Rheumatic heart disease (RHD) is a leading cause of premature death and disability in low-income countries; however, few receive optimal benzathine penicillin G (BPG) therapy to prevent disease progression. We aimed to comprehensively describe the treatment cascade for RHD in Uganda in order to identify appropriate targets for intervention.
Methods and Results
Using data from the Uganda RHD Registry (n=1,504), we identified the proportion of patients in the following care categories: (1) diagnosed and alive as of June 1, 2016, (2) retained in care, (3) appropriately prescribed BPG, and (4) optimally adherent to BPG (>80% of prescribed doses). We used logistic regression to investigate factors associated with retention and optimal adherence. Overall, median (IQR) age was 23 (15–38) years; 69% were female; and 82% had clinical RHD. Median follow-up time was 2.4 (0.9–4.0) years. Retention in care was the most significant barrier to achieving optimal BPG adherence with only 56.9% (95% CI 54.1–59.7%) of living subjects having attended clinic in the prior 56 weeks. Among those retained in care, however, we observed high rates of BPG prescription [91.6% (95% CI 89.1–93.5%)] and optimal adherence (91.4% [95% CI 88.7–93.5]). Younger age, latent disease status, and access to care at a regional center were the strongest independent predictors of retention and optimal adherence.
Conclusions
Our study suggests that improving retention in care—possibly by decentralizing RHD services—would have the greatest impact on uptake of antibiotic prophylaxis among patients with RHD in Uganda.
Despite increased awareness and advocacy in recent years, rheumatic heart disease (RHD) continues to cause substantial disability and premature death among children and young adults in medically-underserved populations around the world1, 2. This preventable disease is characterized by heart valve dysfunction that results from recurrent group A streptococcal infection and acute rheumatic fever (ARF). Secondary prevention of ARF with benzathine penicillin G (BPG) injections slows progression of RHD severity and reduces mortality3–5. Community-based disease registry programs may help ensure compliance with injections but unfortunately have not been widely adopted6.
Uganda is a low-income East African country of 40 million; half are <15 years old and 16% are urban7. Uganda ranks 163rd of 188 countries in Human Development Index8 and adult HIV prevalence is 6.5% (10th highest in the world)7. In 2013, the Uganda Heart Institute, Makerere University, the Joint Clinical Research Center, Case Western Reserve University, and Children’s National Health System formed a collaboration to improve RHD care in Uganda. The collaboration supported a regionalized national registry program that sought to leverage existing HIV infrastructure when possible and to employ tools and “lessons learned” from the scale up of HIV care.
One potential tool to be adapted is the HIV treatment cascade model, which examines discrete and sequential stages necessary to achieve virologic control: HIV testing, knowledge of HIV status, linkage to care, retention/engagement in care, prescription of anti-retroviral therapy (ART), and viral load suppression. The HIV treatment cascade has become a central tool for measuring uptake of HIV care components and informing policy for the UNAIDS 90-90-90 initiative: 90% of persons living with HIV being diagnosed and aware of their status, 90% of those diagnosed with HIV being on ART, and 90% of those on ART being virally-suppressed9.
The treatment cascade concept may help improve RHD care since successful management of chronic RHD faces similar sequential obstacles as in HIV: diagnosis and referral, attendance at regular clinic visits, anti-microbial prescription, and longitudinal adherence. The RHD treatment cascade has not been comprehensively described, except for preliminary findings from our group10. The aims of this study were to describe the treatment cascade among all patients enrolled in the Uganda RHD Registry and to explore clinical and demographic predictors of retention in care and adherence to BPG prophylaxis.
Methods
The Uganda RHD Registry enrolls patients with RHD presenting for clinical care or through echocardiographic screening studies at four regional sites [Uganda Heart Institute (UHI) in Kampala (since 2011), Lubowa (2013), Mbarara (2014), and Gulu (2015)]. The purpose of the registry is to improve clinical care and epidemiologic surveillance of RHD in Uganda. Forms are originally completed on paper and then data is entered into a RedCap platform11. The registry is approved by the Institutional Review Boards of Makerere University School of Medicine (Kampala, Uganda), the Uganda National Council for Science and Technology, and University Hospitals Cleveland Medical Center (Cleveland, OH, USA). All participants older than 18 provide written informed consent. Written parental consent and participant assent are obtained for those younger than 18. This current analysis includes participants enrolled through June 1st, 2016.
Disease categories
Clinicians classified participants at the enrollment visit into the following disease categories:
Acute rheumatic fever (ARF)—defined according to applicable Jones Criteria at the time of diagnosis12, 13 but without evidence of chronic valvular heart disease.
Clinical RHD—defined as patients presenting to clinical attention with symptoms or signs (i.e. murmur) and echocardiographic findings compatible with RHD
Definite latent RHD—identified through echocardiographic screening studies
Borderline latent RHD—identified through echocardiographic screening studies
We defined definite and borderline categories according to the 2012 World Heart Federation guidelines for the diagnosis of latent RHD by echocardiographic screening14.
Treatment cascade categories
We then classified participants into the following sequential treatment cascade categories based on their status as of the audit date, June 1st, 2016. Study nurses and staff attempted to document vital status for all participants by telephone contact with participants or family members. Up to three mobile phone numbers are kept on record for all participants to coordinate care and document health status.
Alive—defined as those without documentation of death. Documentation of death was considered through September 1, 2016, provided that the date of death recorded was on or prior to June 1, 2016. If participants died during the observation period, they were excluded from the subsequent categories.
Retained in care—defined as all living patients (from any disease category) with at least one in-person clinic visit in the past 56 weeks [52 weeks + 4 week grace period for those patients (particularly borderline RHD patients in more remote areas) who might only follow-up once yearly].
Prescribed BPG—defined as all retained participants who had been given a prescription for monthly BPG at last recorded follow-up. Outcomes of “prescribed” and “adherent” excluded those with borderline disease, since our program guidelines do not recommend antibiotic prophylaxis for this group and WHF guidelines offer no recommendation. Those prescribed oral antibiotics in lieu of BPG were counted as “not prescribed”, since the standard of care is intramuscular BPG, which has been shown to be superior to oral penicillin in clinical trials.14
Adherent to BPG—defined as all prescribed participants who had received ≥80% of prescribed BPG doses in the last 12 months [excluding n=8 missing data (1.4% of prescribed)]. The ≥80% definition is widely used as a key performance indicator of adherence15 and was associated with decreased mortality in a previous study from our group5. Adherence was assessed at the most recent follow-up visit using signed administration cards.
Covariates
Participants and/or their caregivers self-reported demographic and socioeconomic covariates at the baseline visit, including age (continuous, and categorized 0–15, 15–25, 25–40, and >40 years), gender, clinic site, distance to nearest health center (km), household number, highest completed education level of participant or participant’s most educated parent if <18 years of age (< or ≥ secondary school), and employment status of participant or participant’s caregiver (yes/no). Study staff used medical records to obtain clinical covariates including New York Heart Association (NYHA) class, medical comorbidity (history of decompensated heart failure, stroke, atrial fibrillation, endocarditis, HIV), and history of valve surgery (repair or replacement). Missingness for these covariates was generally <5%, except for distance to nearest health center (9.1%) and NHYA class (11%).
Statistical analysis
We first described characteristics of the overall registry population and separately by treatment cascade category as median (interquartile range) for continuous variables and number (%) for categorical variables. We compared subjects who had died to those who were alive using t-tests and Wilcoxon rank-sum tests for continuous variables and Fisher’s exact tests for categorical variables. Treatment cascade categories were the outcomes of interest for all analyses.
In the primary analysis and all pre-specified sub-group analyses, we described the total patient number in each treatment cascade category and the proportion (95% CI) of the parent category. As noted above, we excluded borderline RHD participants from the prescribed and adherent categories. We compared differences in the proportion of (A) retained and (B) adherent participants by sub-group using chi-squared or Fisher’s exact tests as appropriate. For the HIV+ subgroup, we performed additional descriptive analyses of immune status and ART use.
We then performed district-level analysis of (A) retention and (B) adherence based on the home address of study participants. For these analyses, we included all subjects with clinical RHD from districts with at least five participants in the denominator. Latent RHD and ARF were excluded due to significant geographic selection bias.
Finally, we constructed unadjusted and multivariable adjusted logistic regression models to explore the association of clinical and demographic variables with the outcomes of (A) retention and (B) adherence. We first examined the entire study population alive at the audit date, and then separately examined clinical RHD participants only. Baseline covariates were used since time-updated measures were not available. In unadjusted analyses, we examined the association of each candidate variable with the outcome of interest. Then for multivariable model selection, we began by forcing categorical age, gender, and disease category into the model. Clinic site was not used in the overall model due to multi-collinearity with disease status, but was used in the clinical RHD models in place of disease category. Additional covariates were selected into the final model using forward selection with retention at p < 0.1. We calculated the AUROC for each model, and used the Hosmer-Lemeshow test to assess goodness of fit. Sensitivity analyses were performed as follows: (1) excluding from the retention models all participants who were enrolled within 90 days of the audit date and (2) excluding from the adherence models all participants who were initially prescribed BPG injections within 90 days of the audit date.
All analyses were performed using STATA 14.2 (StataCorp; College Station, TX, USA) and a p-value of <0.05 was considered statistically significant.
Results
As of June 1, 2016, the Uganda RHD registry consisted of 1504 participants whose characteristics are described in Table 1. Participants with clinical RHD comprised >80% of the study population, whereas ARF represented less than 1%. Age and gender were similar across all treatment cascade categories. Most participants lived within two kilometers of a health center and over half had (or had caregivers with) limited education (completed < secondary school). Among those with clinical RHD, advanced disease and history of morbid complications was common. Median (IQR) follow-up time was 2.4 (0.9–4.0) years and differed by clinic site (median 35 months at UHI vs. 27 months at Lubowa vs. 12 months at Mbarara vs. 9 months at Gulu; p<0.001 for each regional site vs. UHI). Approximately 18% (n=273) of the population died prior to the audit date and were excluded from analyses of the treatment cascade. Patients who died were older (mean age 31 vs. 27 years, p<0.001), lived farther from the nearest health center (median distance 3 vs. 2 km, p=0.002), were more likely to have clinical RHD (97 vs. 78%, p<0.001), and more likely to receive care at the UHI (91 vs. 68%, p<0.001). Twenty-one subjects (1.4%) had transferred follow-up care between study sites prior to the audit date. Nine retained subjects were taking oral antibiotics and thus not categorized as “prescribed”, and only two of these were doing so because of documented penicillin allergy.
Table 1.
Characteristics of the study population overall and separately by treatment cascade category.
| Total Population | Overall (N=1504) | Alive (N=1231) | Retained (N=701) | Prescribed (n=554) | Adherent (n=499) |
|---|---|---|---|---|---|
| Disease Category | |||||
| ARF | 12 (1%) | 11 (1%) | 8 (1%) | 8 (1%) | 7 (1%) |
| Clinical RHD | 1232 (82%) | 966 (78%) | 478 (68%) | 437 (79%) | 394 (79%) |
| Latent Definite | 129 (8%) | 125 (10%) | 119 (17%) | 109 (20%) | 98 (20%) |
| Latent Borderline | 131 (9%) | 129 (11%) | 96 (14%) | n/a | n/a |
| Age | 23 (15–38) | 22 (15–37) | 17 (14–33) | 19 (14–35) | 19 (14–33) |
| Female | 69% | 70% | 68% | 68% | 68% |
| Socioeconomic factors | |||||
| Nearest health center (km) | 2 (1–5) | 2 (1–5) | 2 (1–4) | 2 (1–4) | 2 (1–4) |
| Household number | 6 (4–8) | 6 (4–8) | 6 (4–8) | 6 (4–8) | 6 (4–8) |
| Employed | 25% | 28% | 32% | 28% | 27% |
| Limited Education | 54% | 53% | 51% | 52% | 53% |
| Clinical Site | |||||
| UHI | 72% | 68% | 53% | 60% | 61% |
| Lubowa | 6% | 7% | 12% | 7% | 7% |
| Mbarara | 5% | 4% | 6% | 7% | 6% |
| Gulu | 17% | 21% | 29% | 26% | 26% |
| Clinical RHD Only |
Overall N=1254 |
Alive N=988 |
Retained N=478 |
Prescribed N=437 |
Adherent N=394 |
| NYHA Class | |||||
| I | 22% | 25% | 21% | 21% | 21% |
| II | 50% | 54% | 57% | 57% | 59% |
| III | 19% | 16% | 15% | 16% | 15% |
| IV | 9% | 5% | 7% | 6% | 5% |
| Medical Comorbidity | |||||
| Decompensated HF | 27% | 23% | 24% | 24% | 24% |
| Stroke | 3.7% | 3.6% | 4.0% | 4.2% | 4.4% |
| Atrial Fibrillation | 5.8% | 5.7% | 7.0% | 7.2% | 7.4% |
| Endocarditis | 1.1% | 1.0% | 1.3% | 1.4% | 1.0% |
| HIV | 4.5% | 4.8% | 5.3% | 5.1% | 5.4% |
| Prior Valve Surgery | |||||
| Valve repair | 1.3% | 1.5% | 1.1% | 1.2% | 1.3% |
| Valve replacement | 3.2% | 3.4% | 4.1% | 3.5% | 2.6% |
Data are presented as median (IQR) or % of column]. RHD, rheumatic heart disease; NYHA, New York Heart Association Class; HF, heart failure; HIV, human immunodeficiency virus.
Retention in care was a more significant barrier along the treatment cascade than was prescription of or adherence to BPG injections (Figure 1). Overall, 56.9% (95% CI 54.1–59.7) of living subjects were retained in care, compared to 91.6% (89.1–93.5) prescription of BPG to eligible subjects and 91.4% (88.7–93.5) optimal adherence to BPG. Retention varied substantially by subgroup (Figure 2). Although there were no significant differences by gender, those with clinical RHD were less likely to be retained compared to those in other categories (χ2 p<0.001). Among those with clinical RHD, participants with higher NYHA class were more likely to be retained (χ2 p=0.022). Adherence was similar across all subgroups (Figure 3; all p>0.2). Heat maps of retention and adherence rates by district in displayed in Figures 4 and 5. Supplemental Table 1 shows retention and adherence rates by region.
Figure 1. Overall treatment cascade for patients enrolled in the Uganda RHD registry.
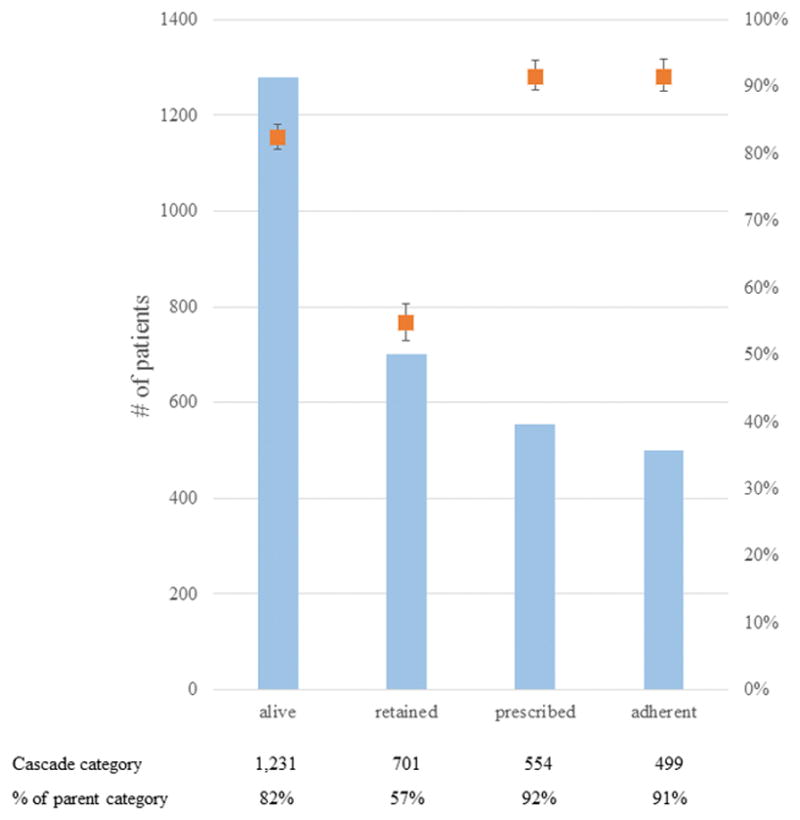
The left axis and blue bars indicate the number of patients in each outcome category of the treatment cascade, while the right axis and orange points indicate the percentage of patients as a proportion of the parent (prior) category. Error bars reflect the 95% confidence interval. All patients in the registry were included to assess outcomes of “alive” and “retained”, but patients with borderline RHD were excluded from assessing the outcomes of “prescribed” and “adherent”.
Figure 2. Subgroup analysis of patient retention.
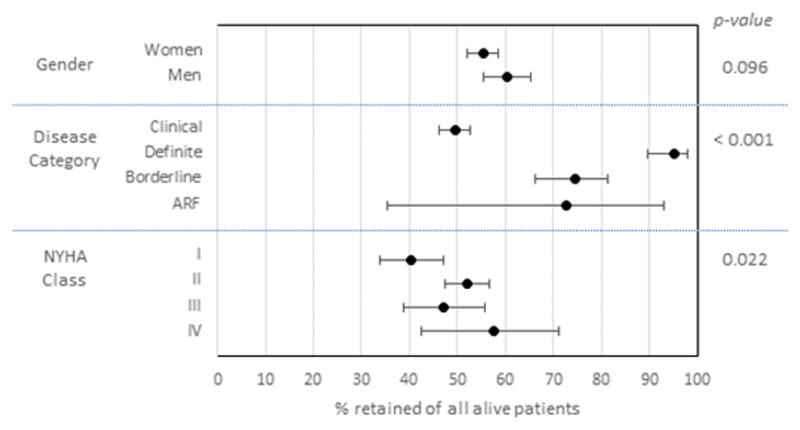
Point estimates represent the percentage of participants retained in care of all those participants who were alive at the end of the observation period. Error bars reflect 95% confidence intervals. Chi-squared p-value is shown on the right.
Figure 3. Subgroup analysis of adherence to prescriptions of prophylactic BPG injections.
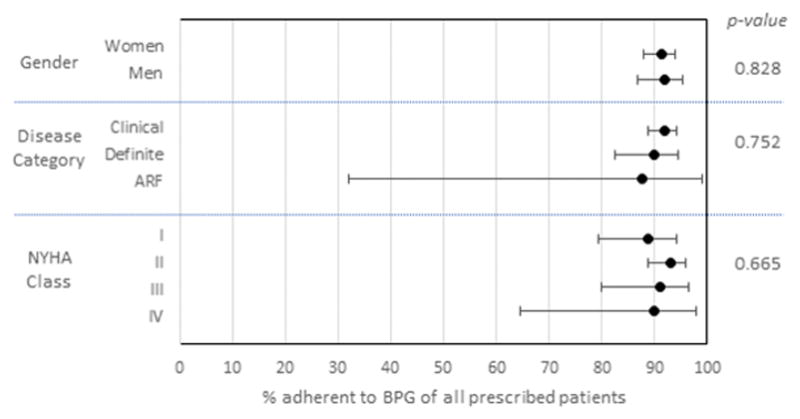
Point estimates represent the percentage of participants who were prescribed BPG who were optimally adherent (>80% of prescribed injections). Error bars reflect 95% confidence intervals. Chi-squared p-value is shown on the right.
Figure 4. Heat map of retention by district.
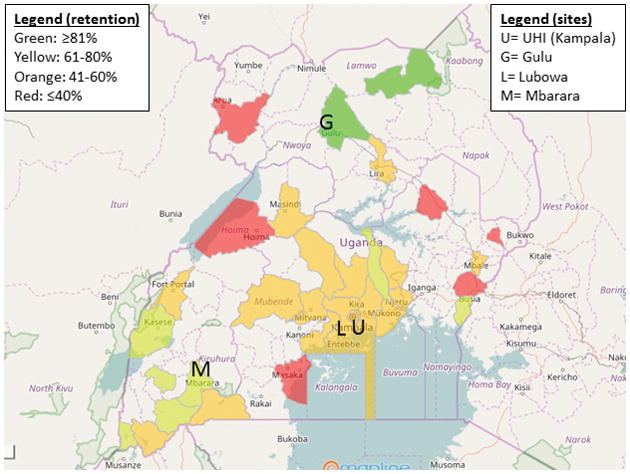
Color coding represents the proportion of patients retained in care. Only districts reporting at least five patients alive at the end of the study were included in this analysis. For reference, the locations of the clinic sites are shown.
Figure 5. Heat map of optimal BPG adherence by district.
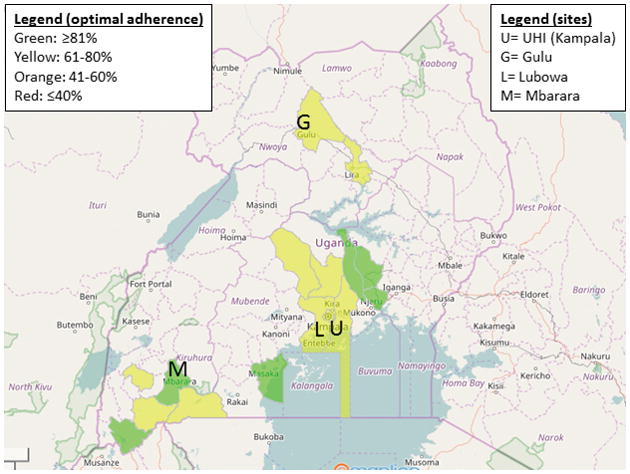
Color coding represents the proportion of patients optimally adherent (>80% of prescribed doses received). Only districts reporting at least five patients prescribed BPG were included in this analysis. The locations of the clinic sites are shown with letters.
The treatment cascade was similar among HIV+ patients compared to the total population (Supplemental Figure 1). Overall, 91% of all registry subjects were aware of their HIV status. Of those with known HIV status, 62 were confirmed HIV+ (4.5 % total; 5.5% of women vs. 2.4% of men, p= 0.012 for gender comparison). Patients with HIV were not more likely to have died compared to those without HIV (p=0.309). Median (IQR) age was 41 (31–46), with only 6 patients being less than 25 years old and 2 being less than 15 years old. The median (IQR) CD4+ count at enrollment was 365 (237–608) cells/ml and CD4+ at diagnosis was 230 (112–374) cells/ml. Forty-eight participants (77% of HIV+) had documented antiretroviral treatment (ART) status, and 90% (95% CI 77–96%) of these participants were on ART.
Predictors of retention in care are shown in Table 2 (full cohort) and Supplemental Table 2 (clinical RHD only). Among all living subjects in the full cohort, younger age, latent disease status, closer distance to health center, being employed (or having an employed caregiver), and having more advanced education were associated with higher odds of retention. Among clinical RHD participants, the effect of age and employment were attenuated after adjustment for other factors, and the strongest predictor of retention was whether the participant was enrolled at one of the three regional centers (vs. UHI). Other variables associated with higher retention among clinical RHD participants were closer distance to the nearest health center and more advanced education. As in the subgroup analysis above, higher NYHA class was associated with improved retention in unadjusted analyses. This relationship was somewhat attenuated after adjustment for possible confounders; however, those with mild symptoms (NYHA II) were more likely to be retained compared to those with little to no symptoms (NYHA I). Although HIV infection was also associated with borderline higher retention in unadjusted analyses (p=0.058), this was attenuated after multivariable adjustment and not kept in the final model.
Table 2.
Association of demographic variables with retention in care among all living subjects (n=1,231).
| Unadjusted | Multivariable | |||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95% CI) | p | |
| Age | ||||
| 0–15 years | REF | REF | ||
| 15–25 years | 0.49 (0.36–0.68) | <0.001 | 0.56 (0.38–0.82) | 0.003 |
| 25–40 years | 0.38 (0.27–0.54) | <0.001 | 0.49 (0.32–0.75) | 0.001 |
| >40 years | 0.32 (0.23–0.44) | <0.001 | 0.46 (0.30–0.70) | <0.001 |
| Female (vs male) | 0.81 (0.63–1.04) | 0.096 | 0.99 (0.74–1.34) | 0.964 |
| Disease category | ||||
| Clinical | REF | REF | ||
| Latent Definite | 20.2 (8.8–46.4) | <0.001 | 14.3 (6.1–33.5) | <0.001 |
| Latent Borderline | 2.97 (1.96–4.50) | <0.001 | 2.30 (1.40–3.78) | 0.001 |
| Acute Rheumatic Fever | 2.72 (0.72–10.3) | 0.141 | 2.76 (0.54–14.1) | 0.224 |
| Distance to the nearest health centre (per km) | 0.91 (0.88–0.94) | <0.001 | 0.94 (0.91–0.98) | 0.001 |
| Household size (per person) | 0.96 (0.93–0.99) | 0.038 | -- | -- |
| Employed or Employed Caregiver (vs. not) | 1.6 (1.2–2.1) | <0.001 | 1.42 (1.03–1.96) | 0.030 |
| Limited Education (vs. more advanced education) | 0.84 (0.67–1.05) | 0.130 | 0.69 (0.53–0.92) | 0.011 |
Bold print represents p<0.05. Hosmer-Lemeshow test for goodness of fit (p=0.89). AUROC curve 0.717 (95%CI 0.686–0.747).
Predictors of adherence to BPG are shown in Table 3 and Supplemental Table 3. Younger age was again independently associated with better adherence among all retained subjects in the full cohort, but the effect was not statistically significant in the clinical RHD subgroup. Latent disease status and limited education were associated with better adherence after adjustment for age and other confounders in the full cohort. In the clinical RHD subgroup, limited education was associated with better adherence and history of prior valve surgery was associated with worse adherence.
Table 3.
Association of demographic variables with optimal adherence to penicillin among subjects retained in care excluding borderline disease (n=597).
| Unadjusted | Multivariable | |||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95% CI) | p | |
| Age | ||||
| 0–15 years | REF | REF | ||
| 15–25 years | 1.14 (0.59–2.23) | 0.695 | 1.26 (0.63–2.51) | 0.515 |
| 25–40 years | 0.47 (0.25–0.86) | 0.014 | 0.42 (0.21–0.85) | 0.015 |
| >40 years | 0.51 (0.27–0.97) | 0.040 | 0.43 (0.21–0.89) | 0.022 |
| Female (vs male) | 0.74 (0.45–1.21) | 0.227 | 0.84 (0.50–1.40) | 0.506 |
| Disease category | ||||
| Clinical | REF | REF | ||
| Latent Definite | 0.90 (0.53–1.53) | 0.698 | 0.50 (0.26–0.93) | 0.029 |
| Acute Rheumatic Fever | 1.35 (0.16–11.1) | 0.780 | 1.23 (0.14–11.0) | 0.853 |
| Distance to the nearest health centre (per km) | 1.01 (0.94–1.08) | 0.810 | -- | -- |
| Household size (per person) | 1.07 (0.99–1.16) | 0.105 | -- | -- |
| Employed or Employed Caregiver (vs. not) | 0.54 (0.35–0.86) | 0.009 | -- | -- |
| Limited Education (vs. more advanced education) | 1.75 (0.12–2.74) | 0.013 | 1.70 (1.06–2.74) | 0.028 |
Bold print represents p<0.05. Hosmer-Lemeshow test for goodness of fit (p=0.75). AUROC curve 0.651 (95% CI 0.595–0.707).
Sensitivity analyses were performed to determine the influence of short follow-up time on retention and adherence data. When patients enrolled within 90 days of the audit date were excluded, results of the overall treatment cascade were similar (Supplemental Figure 2). In the retention model, similar associations were seen except that the odds ratio for latent disease increased substantially [OR 25.4 (95% CI 7.9–82.1) and OR 11.0 (95% CI 4.3–28.2) for definite and borderline categories, respectively], and the AUROC also increased from 0.717 (95% CI 0.686–0.747) to 0.750 (95% CI 0.729–0.780). When patients starting prophylaxis within 90 days of the audit date were excluded from the adherence model, results were very similar except the association with latent disease was no longer statistically significant [OR 0.59 (95% CI 0.31–1.13), p=0.111].
Discussion
This novel approach to assessing the quality of care for RHD patients in Uganda reveals that retention in care is the most significant barrier to achieving optimal BPG adherence, which can prevent ARF recurrence, progression of RHD, and death3–6. We observed high rates of adherence among those retained in care, consistent with the HIV literature16 suggesting that medication adherence per se is not a significant problem for RHD patients in Africa. We have previously reported on the treatment cascade early after the RHD registry was adopted10 and for children with latent RHD in Gulu who participated in echocardiographic screening studies17. Here, we extend our analysis to the entire Uganda RHD registry with nearly 2.5 years of median follow-up time and report on independent predictors of retention and adherence.
Mortality, retention, and BPG adherence in our study were similar to the overall Global Rheumatic Heart Disease registry (REMEDY)4, 18, which enrolled patients with clinical RHD from predominantly low-income south and east African countries and contained a population with similar demographics and functional status. Of note, the global study had a more lenient definition of retention: one repeat visit anytime within the two-year study (vs. 56 weeks in our study). Our estimates of adherence were additionally biased downward by categorizing oral antibiotic use as non-adherent (although there were only nine of these subjects) and by only using objective documentation of adherence rather than self-report.
The proportions of patients within each component of the RHD treatment cascade were also similar to analogous outcomes from the CDC HIV care continuum19, which were below 90-90-90 initiative goals. Progress is being made, but more improvement in HIV care is needed to reach these goals. In sub-Saharan Africa, approximately 51% of people living with HIV were on ART in 2015, compared to about 21% in 201020. Retention is a critical factor, with a recent meta-analysis estimating that only 65% of Africans starting ART are retained in care at least 36 months21.
Interestingly, our data support prior reports of a negative correlation between HIV infection and RHD, although the treatment cascade outcomes were similar regardless of HIV status. The prevalence of HIV in our cohort (4.5% overall, <1% age 15–24) was significantly lower than the general population of Uganda (7.4% overall, 3.7% age 15–24).20 Conversely, an echocardiographic screening study by our group22 found that the prevalence of RHD in a cohort of HIV-infected children was less than the general population of school-aged children in Kampala2,3. Children with HIV may have more engagement with the health system, leading to better surveillance for and treatment of GAS pharyngitis. Other proposed mechanisms include the antimicrobial and anti-inflammatory effects of cotrimoxazole prophylaxis21. Larger prospective studies are needed to confirm the role of these possible mechanisms.
Significant barriers to care utilization exist in sub-Saharan Africa, including lack of transportation, poor roads/infrastructure, poverty, limited education/literacy, weather during rainy season, scheduling conflicts, drug stock-outs, and poor provider awareness or communication of health problems. Our study demonstrated that living farther away from local and regional health care centers is a barrier to retention, as reflected in the multivariable model and the geographic heat map of retention rates. Since our analysis adjusted for distance to nearest health center, improved retention at regional sites is also likely attributable to more staff, funding, and ancillary resources per-capita dedicated to tracking patients—resulting from our initiative to decentralize RHD care. For example, HIV counselors have been repurposed to improve retention and adherence for RHD patients at the Lubowa site. Similarly, the HIV literature has demonstrated poorer retention with larger clinic size23 and with care in higher tiers of the health system24, further supporting the need for decentralized services. Although our heat map of retention and adherence rates suggests geographic disparities in the treatment cascade, our study was limited by the number of patients coming from more remote districts. As our national registry program is rolled out to additional regional centers and more patients are enrolled from rural districts, we will be able to conduct a more robust analysis of geographic disparities in RHD care in Uganda.
Younger age was independently associated with better retention and adherence in our study. The effect of age on adherence may be influenced by strong linkages between RHD clinics and schools where echocardiographic screening was performed. For example, in Gulu, there is an active pediatric support group and dedicated clinical resources aimed at keeping children engaged in follow-up care25. The age effect was also seen, however, in a previous report from the UHI5. These findings contrast with the HIV literature since a large multi-center African study suggested that ART adherence improves with age26.
Patients and caregivers managing HIV or RHD face a similar bottleneck: retention in care. Thus, policy makers working to improve RHD control should focus on this step in the cascade. Since there are no tested interventions known to improve treatment cascade metrics for RHD in Africa, initial efforts might focus on strategies proven to work for HIV care. Specific interventions that appear to be most effective in HIV include weekly SMS reminders, treatment supporters, and enhanced counseling27. A large meta-analysis has also highlighted the benefits of decentralizing care and task shifting, demonstrating a more favorable effect for community-based vs. clinic-based ART programs on engagement in care in low- and middle-income countries28. Although both community- and clinic-based interventions demonstrated similar effects on adherence, virologic suppression, and mortality in this meta-analysis, interim data from the contemporary SEARCH study in east Africa suggest that community-based testing and treatment may lead to even greater improvements in ART coverage and viral suppression that meet 90-90-90 goals29. RHD control programs typically receive limited funding due to competing public health priorities, so cost-effectiveness and local sustainability are important. We recommend decentralizing services and adapting interventions like those above to support RHD care, focusing on patients most at risk for poor treatment cascade outcomes.
Strengths of this study include the large, extensively characterized sample size from Kampala and multiple regional centers and the relatively low proportion of incomplete or missing data. The study also has several limitations. Due to multi-collinearity resulting from certain sites having a high proportion of latent RHD patients enrolled from echocardiographic screening studies, the independent effects of disease category and clinic site could not be fully explored. Second, availability of time-updated clinical variables such as NYHA status would have strengthened the analysis. Third, although we made repeated attempts to contact those who were lost to follow-up, some of these may have died or transferred care causing misclassification. For example, a large meta-analysis found that 24% of HIV+ subjects lost to follow-up had self-transferred care and 34% had died30. Fourth, it is possible that differences in follow-up time at the UHI vs. regional centers may explain some of our findings regarding decentralized care, which should be clarified in subsequent analyses of the registry with longer follow-up. Finally, because Uganda has benefited from targeted interventions to improve RHD care, our results may not be generalizable to other countries with even less investment in RHD.
Conclusions
Based on this analysis of the RHD treatment cascade, the greatest opportunity to improve the uptake of adequate antibiotic prophylaxis in patients would be improving retention in longitudinal care. Demographic variables and clinical site influenced retention and BPG adherence more than clinical variables. Future studies should test the implementation of interventions to improve retention among those at highest risk, including decentralization of RHD care and BPG prophylaxis.
Supplementary Material
What is Known?
The burden of disability and premature death from rheumatic heart disease is highest among low-income countries, where health systems are ill-equipped to care for chronic non-communicable diseases.
Secondary prophylaxis injections of Benzathine penicillin G slows progression of rheumatic heart disease and reduces mortality, but poor adherence over time is common in sub-Saharan African countries.
The treatment cascade model has been used effectively to evaluate HIV/AIDS control programs worldwide but may also be applicable to other chronic diseases that face similar obstacles to care.
What the Study Adds?
This large study of over 1,500 patients from a national rheumatic heart disease registry in Uganda highlights that efforts to improve uptake of penicillin prophylaxis for RHD should focus on retaining patients in care.
Younger age, latent (vs. clinically symptomatic) disease, and close access to care at local health centers and regional centers of excellence were the strongest independent predictors of retention in care and adherence to penicillin.
Acknowledgments
The authors would like to acknowledge the following collaborators, nurses and personnel who contributed to formation of and maintenance of the Uganda RHD Registry: Rose Akech, Geoffrey Amone, Emily Atukunda, Alyssa Dewyer, Jenipher Kamarembo, James Kayima, Samalie Kitooleko, Peninah Komugabe, Victor Musiime, Peter Mugyenyi, Annette Musana, Phyllis Mwesigwa, Lydia Namuli, Ian Natuhurira, Theresa Ndagire, Florence Odongo, Matthew Odera, and Martin Ojok. Author Contributions: All authors contributed to study design and oversight of data collection within the Uganda RHD registry collaboration. SRM and CTL analyzed and interpreted the data and drafted the manuscript. CTL performed the statistical analysis. All authors critically revised the manuscript for important intellectual content. CTL and EO supervised the study.
Sources of Funding: This study was supported with a grant from the Medtronic Global Health Foundation. CTL is supported by grants from the National Institutes of Health (K23 HL123341) and the Wolf Family Foundation. EO is supported by the DELTAS Africa Initiative grant # DEL-15-011 to THRiVE-2. CTL and EO had full access to all the data and had final responsibility for the decision to submit for publication.
Footnotes
Disclosures: CTL has received honoraria from Gilead Sciences. SRM, TOA, AB, MAC, MRK, CK, PL, GM, DN, JR, CS, RAS, AS, DIS, IS, and EO have no conflicts to disclose relevant to this manuscript.
References
- 1.Sika-Paotonu D, Beaton A, Raghu A, Steer A, Carapetis J. Acute Rheumatic Fever and Rheumatic Heart Disease. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes : Basic Biology to Clinical Manifestations. Oklahoma City (OK): 2016. [Google Scholar]
- 2.Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Riberio AL, Sable CA, Steer AC, Naghavi M, Mokdad A, Murray CJ, Vos T, Carapetis JR, Roth GA. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. The New England journal of medicine. 2017;377:713–22. doi: 10.1056/NEJMoa1603693. [DOI] [PubMed] [Google Scholar]
- 3.Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, Taubert KA. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119:1541–51. doi: 10.1161/CIRCULATIONAHA.109.191959. [DOI] [PubMed] [Google Scholar]
- 4.Zuhlke L, Karthikeyan G, Engel ME, Rangarajan S, Mackie P, Cupido-Katya Mauff B, Islam S, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al-Kebsi MM, Hugo-Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode-Thomas F, Yilgwan CC, Amusa GA, Ige O, Okeahialam B, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani MU, Ogah OS, Elhassan TO, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Clinical Outcomes in 3343 Children and Adults With Rheumatic Heart Disease From 14 Low- and Middle-Income Countries: Two-Year Follow-Up of the Global Rheumatic Heart Disease Registry (the REMEDY Study) Circulation. 2016;134:1456–1466. doi: 10.1161/CIRCULATIONAHA.116.024769. [DOI] [PubMed] [Google Scholar]
- 5.Okello E, Longenecker CT, Beaton A, Kamya MR, Lwabi P. Rheumatic heart disease in Uganda: predictors of morbidity and mortality one year after presentation. BMC Cardiovasc Disord. 2017;17:20. doi: 10.1186/s12872-016-0451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remenyi B, Carapetis J, Wyber R, Taubert K, Mayosi BM World Heart F. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat Rev Cardiol. 2013;10:284–92. doi: 10.1038/nrcardio.2013.34. [DOI] [PubMed] [Google Scholar]
- 7.The World Factbook. Uganda: U.S. Central Intelligence Agency; [Accessed October 19, 2017]. https://www.cia.gov/library/publications/the-world-factbook/geos/ug.html. [Google Scholar]
- 8.United Nations Development Programme. New York, New York: 2016. Human Development Report. [Google Scholar]
- 9.UNAIDS Joint United Nations Programme on HIV/AIDS. Geneva, Switzerland: 2014. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. [Google Scholar]
- 10.Longenecker C, Okello E, Aliku T, Beaton A, Mirembe G, Webel A, Lwabi P, Kityo C, Musiime V, Sable C, Kamya M, Costa M, Salata R, Simon D. Abstract 18798: Application of the HIV/AIDS Treatment Cascade Model to Rheumatic Heart Disease in Uganda. Circulation. 2014;130:A18798–A18798. [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. Jama. 1992;268:2069–73. [PubMed] [Google Scholar]
- 13.Gewitz MH, Baltimore RS, Tani LY, Sable CA, Shulman ST, Carapetis J, Remenyi B, Taubert KA, Bolger AF, Beerman L, Mayosi BM, Beaton A, Pandian NG, Kaplan EL American Heart Association Committee on Rheumatic Fever E, Kawasaki Disease of the Council on Cardiovascular Disease in the Y. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806–18. doi: 10.1161/CIR.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 14.Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, Mocumbi AO, Mota C, Paar J, Saxena A, Scheel J, Stirling J, Viali S, Balekundri VI, Wheaton G, Zuhlke L, Carapetis J. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease--an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Dassel JL, Fittock MT, Wilks SC, Poole JE, Carapetis JR, Ralph AP. Adherence to secondary prophylaxis for rheumatic heart disease is underestimated by register data. PLoS One. 2017;12:e0178264. doi: 10.1371/journal.pone.0178264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, Rachlis B, Wu P, Cooper C, Thabane L, Wilson K, Guyatt GH, Bangsberg DR. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. Jama. 2006;296:679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 17.Longenecker CT, Aliku T, Beaton A, Scheel A, Okello E, Perlman L, Sable C, Lwabi P. PM290 Treatment Cascade Quality Metrics for Children With Latent Rheumatic Heart Disease in Uganda. Global Heart. 2016;11:e119–e120. [Google Scholar]
- 18.Zuhlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi P, Al-Kebsi MM, Hugo-Hamman C, Sheta SS, Haileamlak A, Daniel W, Goshu DY, Abdissa SG, Desta AG, Shasho BA, Begna DM, ElSayed A, Ibrahim AS, Musuku J, Bode-Thomas F, Okeahialam BN, Ige O, Sutton C, Misra R, Abul Fadl A, Kennedy N, Damasceno A, Sani M, Ogah OS, Olunuga T, Elhassan HH, Mocumbi AO, Adeoye AM, Mntla P, Ojji D, Mucumbitsi J, Teo K, Yusuf S, Mayosi BM. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study) Eur Heart J. 2015;36:1115–22a. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, Skarbinski J, Higa DH, Prejean J, Frazier EL, Patel R, Huang P, An Q, Song R, Tang T, Valleroy LA. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV--United States, 2011. MMWR Morb Mortal Wkly Rep. 2014;63:1113–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Global AIDS Update 2016. UNAIDS Joint United Nations Programme on HIV/AIDS. Geneva, Switzerland: 2016. [Google Scholar]
- 21.Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. Journal of acquired immune deficiency syndromes. 2015;69:98–108. doi: 10.1097/QAI.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleason B, Mirembe G, Namuyonga J, Okello E, Lwabi P, Lubega I, Lubega S, Musiime V, Kityo C, Salata RA, Longenecker CT. Brief Report: Prevalence of Latent Rheumatic Heart Disease Among HIV-Infected Children in Kampala, Uganda. Journal of acquired immune deficiency syndromes. 2016;71:196–9. doi: 10.1097/QAI.0000000000000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimsrud A, Balkan S, Casas EC, Lujan J, Van Cutsem G, Poulet E, Myer L, Pujades-Rodriguez M. Outcomes of antiretroviral therapy over a 10-year period of expansion: a multicohort analysis of African and Asian HIV programs. Journal of acquired immune deficiency syndromes. 2014;67:e55–66. doi: 10.1097/QAI.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 24.Fatti G, Grimwood A, Bock P. Better antiretroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS One. 2010;5:e12888. doi: 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheel A, Beaton A, Okello E, Longenecker CT, Otim IO, Lwabi P, Sable C, Webel AR, Aliku T. The impact of a peer support group for children with rheumatic heart disease in Uganda. Patient Educ Couns. 2017 Jul 11; doi: 10.1016/j.pec.2017.07.006. pii: S0738–3991(17)30419–6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Bijker R, Jiamsakul A, Kityo C, Kiertiburanakul S, Siwale M, Phanuphak P, Akanmu S, Chaiwarith R, Wit FW, Sim BL, Boender TS, Ditangco R, Rinke De Wit TF, Sohn AH, Hamers RL. Adherence to antiretroviral therapy for HIV in sub-Saharan Africa and Asia: a comparative analysis of two regional cohorts. J Int AIDS Soc. 2017;20:1–10. doi: 10.7448/IAS.20.1.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills EJ, Lester R, Thorlund K, Lorenzi M, Muldoon K, Kanters S, Linnemayr S, Gross R, Calderon Y, Amico KR, Thirumurthy H, Pearson C, Remien RH, Mbuagbaw L, Thabane L, Chung MH, Wilson IB, Liu A, Uthman OA, Simoni J, Bangsberg D, Yaya S, Bärnighausen T, Ford N, Nachega JB. Interventions to promote adherence to antiretroviral therapy in Africa: a network meta-analysis. The Lancet HIV. 2014;1:e104–e111. doi: 10.1016/S2352-3018(14)00003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nachega JB, Adetokunboh O, Uthman OA, Knowlton AW, Altice FL, Schechter M, Galarraga O, Geng E, Peltzer K, Chang LW, Van Cutsem G, Jaffar SS, Ford N, Mellins CA, Remien RH, Mills EJ. Community-Based Interventions to Improve and Sustain Antiretroviral Therapy Adherence, Retention in HIV Care and Clinical Outcomes in Low- and Middle-Income Countries for Achieving the UNAIDS 90-90-90 Targets. Curr HIV/AIDS Rep. 2016;13:241–55. doi: 10.1007/s11904-016-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen M, Balzer L, Kwarsiima D, Sang N, Chamie G, Ayieko J, Kabami J, Owaraganise A, Liegler T, Mwangwa F, Kadede K, Jain V, Plenty A, Brown L, Lavoy G, Schwab J, Black D, van der Laan M, Bukusi EA, Cohen CR, Clark TD, Charlebois E, Kamya M, Havlir D. Association of Implementation of a Universal Testing and Treatment Intervention With HIV Diagnosis, Receipt of Antiretroviral Therapy, and Viral Suppression in East Africa. Jama. 2017;317:2196–2206. doi: 10.1001/jama.2017.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zurcher K, Mooser A, Anderegg N, Tymejczyk O, Couvillon MJ, Nash D, Egger M IeDea and consortia M. Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Tropical medicine & international health : TM & IH. 2017;22:375–387. doi: 10.1111/tmi.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


