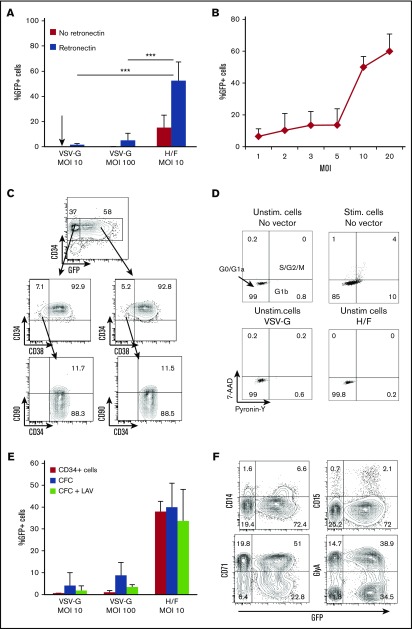
Figure 2.
H/F-LVs allow high-level transduction of unstimulated CD34+cells without altering their immature phenotype. (A) Freshly isolated hCD34+ cells were transduced with H/F-LVs or VSV-G-LVs at indicated MOIs in the presence or absence of retronectin. No cytokines were added. Three days after transduction, the cells were evaluated for the percentage of GFP+hCD34+ cells by FACS (mean ± SD; n = 6). (B) Unstimulated hCD34+ cells were transduced with H/F-LVs at increasing MOIs in the presence of retronectin. Three days after transduction, the cells were analyzed for the percentage of GFP+hCD34+ cells by FACS (mean ± SD; n = 6). (C) Surface marking of very immature progenitors (CD34+CD38–CD90+) for the transduced (GFP+) and untransduced (GFP–) cells. The arrows indicate the gated areas. (D) Cell cycle progression was monitored by simultaneously visualizing the RNA (Pyronine-Y) and DNA (7-AAD) content of the hCD34+ cells 3 days after transduction. The percentages of cells in the G0/G1a, G1b, and S/G2/M phase of the cell cycle are indicated in the dot blots. (E) Comparison between the percentage of GFP+ unstimulated (Unstim) CD34+ cells and the percentage of GFP+ myeloid colonies (CFCs) derived from these transduced CD34+ cells. In parallel, the CFC assay was performed in the presence of the RT inhibitor lamivudine (LAV) to exclude rescue of transduction resulting from H/F-LV bound to the cell surface upon cytokine stimulation (mean ± SD; n = 4). (F) Surface staining for the different transduced myeloid colonies derived from unstimulated transduced hCD34+ cells (GlyA for erythrocytes; CD15 and CD14 for granulocytes, monocytes, and macrophages; CD71 for immature erythrocyte progenitors). Data in panels C, D, and F are representative of 3 independent experiments. Statistical analysis for comparison of VSV-G-LVs vs H/F-LVs was performed by using a paired Student t test. ***P < .001. Stim, stimulated.