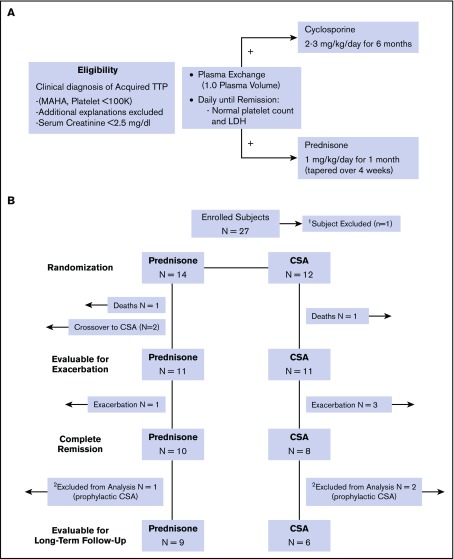
Figure 1.
Treatment plan and description of outcomes for enrolled subjects. (A) The eligibility and treatment schema for the study are shown. Eligible patients were randomly assigned to prednisone or CSA 1:1 in a prespecified manner. (B) The eligibility and treatment schema for the study are shown. A total of 11 subjects in each arm were evaluable for the primary outcome of exacerbation. Of the 18 subjects evaluable for long-term relapse rate, 3 subjects (1 in the prednisone arm, 2 in the CSA arm) elected to receive prophylactic therapy and were not evaluable for relapse.