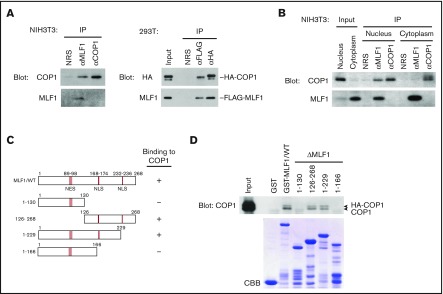
Figure 1.
Myeloid leukemia-associated MLF1 specifically interacts with COP1 in the nucleus. (A) Specific interaction between endogenous MLF1 and COP1 proteins (left panels). Endogenous MLF1 and COP1 proteins were immuno-coprecipitated from the NIH3T3 cell lysate shown at the top and analyzed by immunoblotting with antibodies to COP1 (upper panel) and MLF1 (lower panel). Specific interaction between ectopically expressed MLF1 and COP1 proteins (right panels). The 293T cells were cotransfected with FLAG-tagged MLF1 and HA-tagged COP1. Ectopic MLF1 and COP1 proteins were immuno-coprecipitated from cell lysates shown at the top. The immune complex was analyzed by immunoblotting with antibodies to an HA epitope (upper panel) and MLF1 (lower panel). NRS, normal rabbit serum. (B) Nuclear and cytoplasmic fractions from NIH3T3 cells were separated by sequential elutions with digitonin-containing buffer and analyzed by immunoblotting using antibodies to COP1 and MLF1. (C) Schematic representation of MLF1 deletion mutants. The results of COP1 binding are summarized on the right. (D) The region of MLF1 involved in binding to COP1 in vitro. GST-control, GST-MLF1 WT, and GST-MLF1 mutant fusion proteins shown at the top of the panel were incubated with 293T cell lysates containing HA-tagged COP1 proteins. Bound proteins were detected by immunoblotting with an antibody to COP1. The amounts of GST proteins absorbed on the beads were evaluated by Coomassie brilliant blue (CBB) staining.