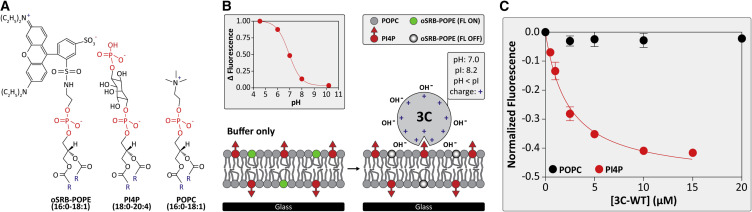
Figure 4.
Binding of a Mono-phosphorylated PIP to 3C in the Context of a Membrane
(A) Chemical structures of the supported lipid bilayer (SLB) components; pH-sensitive oSRB-POPE; PI4P; and POPC. “R” represents the fatty acyl chains.
(B) Schematic diagram illustrating the principles of the SLB-binding experiment. In the absence of 3C, the fluorescent probe is in its “on state” (left). Upon binding to the membrane, the interfacial potential is increased, causing the fluorescent probe to switch to its “off state” (right). The pH-response curve of the fluorescent probe in a bilayer containing 92 mol% POPC, 7.5 mol% PI4P, and 0.5 mol% oSRB-POPE is shown.
(C) 3C binding to PI4P-containing SLBs. Change in fluorescence intensity was observed as a function of 3C concentration, which was normalized to a reference channel. Shown is a hyperbolic fit of the dataset. The apparent dissociation constant for 3C-PI4P interaction is 2.4 ± 0.2 μM. 3C was unable to bind to pure POPC membranes. Error bars represent the SEM (n = 3).