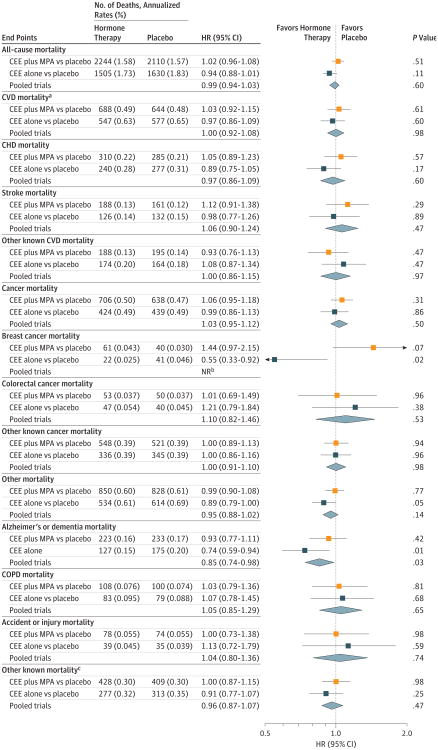
Figure 2. Mortality Outcomes in the Women's Health Initiative Hormone Therapy Trials During the 18-Year Cumulative Follow-up.
The 18-year follow-up is cumulative, indicating the intervention plus extended postintervention phases of the 2 trials (median, 17.7 [interquartile range {IQR}, 16.6-18.6] years in the conjugated equine estrogens [CEE] plus medroxy progesterone acetate [MPA] trial; median, 17.7 [IQR, 16.5-18.7] years in the CEE-alone trial; and median,17.7 [IQR,16.6-18.6] years in the pooled analysis).
aCardiovascular disease (CVD) mortality includes deaths due to myocardial infarction, coronary heart disease, stroke, heart failure, peripheral vascular disease, venous thromboembolism, and other major causes of CVD death.
bThe P value corresponding with a test of heterogeneity between trial-specific hazard ratios (HRs) was .05 or less; therefore, the pooled estimate and HR (95% CI) are not reported.
cIndicates other mortality outcomes that were known but were not due to Alzheimer disease or other dementia, chronic obstructive pulmonary disease (COPD), or accident or injury.