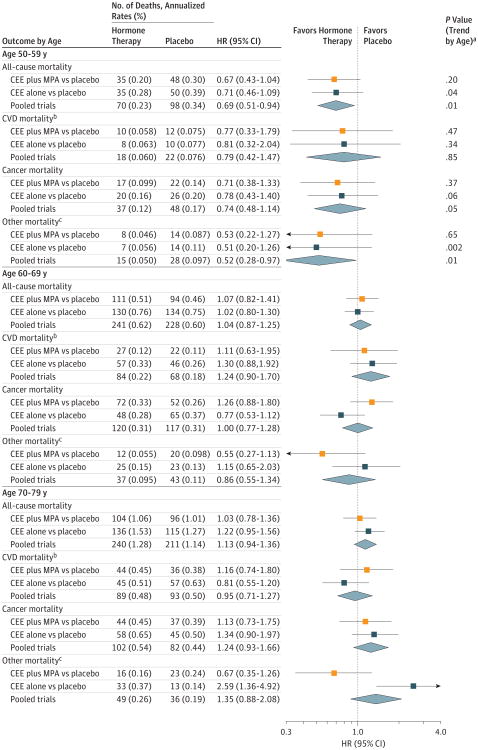
Figure 3. Mortality Outcomes During the Intervention Phase According to 10-Year Age Groups at Randomization.
Reported values indicate the duration of follow-up for the intervention phase (median, 5.6 [interquartile range {IQR}, 4.9-6.5] years in the conjugated equine estrogens [CEE] plus medroxyprogesterone acetate [MPA] trial; median, 7.2 [IQR, 6.5-8.2] years in the CEE-alone trial; and median, 6.3 [IQR, 5.3-7.3] years in the pooled analysis). Age groups indicate participant ages at randomization. HR indicates hazard ratio.
aP values based on a test for trend of interaction between the randomization group and the age group.
bCardiovascular disease (CVD) mortality includes deaths due to myocardial infarction, coronary heart disease, stroke, heart failure, peripheral vascular disease, venous thromboembolism, and other major causes of CVD death.
c Indicates mortality outcomes not due to CVD or cancer.