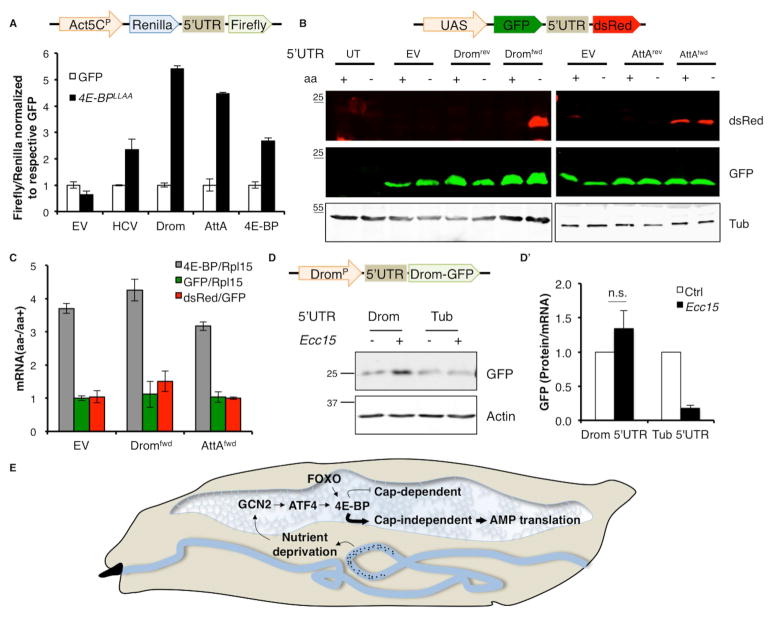
Fig. 4. 4E-BP enhances the translation of AMPs.
4A. Bicistronic reporter (schematic on top) assays with 5’UTRs indicated on X-axis in S2 cells overexpressing either GFP as a control (white) or 4E-BPLLAA (black). Firefly luciferase activity of each sample is normalized to Renilla luciferase activity and all samples are normalized to their respective control (GFP). Error bars indicate standard deviation.
4B. Western blot analysis of the fluorescent bicistronic reporter (schematic on top) with Drosomycin (Drom) and Attacin A (AttA) 5’UTR in forward (fwd) or reverse (rev) orientation in S2 cells grown in either complete media (control) or media lacking amino acids (aa). (UT= untransfected control, EV= empty vector).
4C. qPCR analysis of S2 cells expressing the Dromfwd and AttAfwd 5’UTR fluorescent bicistronic reporter (as in 4B) subjected to amino acid (aa) deprivation. Data represents fold changes in aa deprived cells w.r.t. cells grown in complete media, with mRNA normalized as indicated in legend.
4D. Western blot of hemolymph from transgenic larvae expressing monocistronic reporters (schematic on top) driven by the Drosomycin promoter (DromP) with indicated 5’UTRs (Drom= Drosomycin, Tub= Tubulin) infected with Ecc15. The Drosomycin-GFP (Drom-GFP) fusion protein is detected using an anti-GFP antibody. 4D’ quantifies translation of Drom-GFP.relative to Drom-GFP mRNA levels.
4E. A model for 4E-BP activation and function during enteric infection in larvae. In response to enteric infections by pathogens (indicated by black dots), GCN2 is activated in the fat body likely due to nutrient deprivation. GCN2 activation in the fat body leads to ATF4 synthesis and subsequent transcriptional induction of 4e-bp. FOXO also contributes to 4e-bp induction. Once induced, 4E-BP itself is translated cap-independently and blocks cap-dependent translation of most cellular transcripts. In doing so, it promotes (indicated by bold arrow) cap-independent translation of AMP transcripts. Such bias in cellular translation by 4E-BP is required to drive AMP synthesis during the infection, thus explaining the role of 4E-BP in the innate immune response.
Error bars indicate standard error from 3 independent experiments.