Abstract
Background
The implementation of Medicare Part D on January 1, 2006 required all adults who were dually enrolled in Medicaid and Medicare (dual eligibles) to transition prescription drug coverage from Medicaid to Medicare Part D. Changes in payment systems and utilization management along with the loss of Medicaid protections had the potential to disrupt medication access, with uncertain consequences for dual eligibles with HIV who rely on consistent prescription coverage to suppress their HIV viral load.
Objective
To estimate the effect of Medicare Part D on self-reported out-of-pocket prescription drug spending, AIDS Drug Assistance Program (ADAP) use, antiretroviral adherence, and HIV viral load suppression among dual eligibles with HIV.
Methods
Using 2003–2008 data from the Women’s Interagency HIV Study, we created a propensity score matched cohort and used a difference-in-differences approach to compare dual eligibles’ outcomes pre- and post-Medicare Part D to those enrolled in Medicaid alone.
Results
Transition to Medicare Part D was associated with a sharp increase in the proportion of dual eligibles with self-reported out-of-pocket prescription drug costs, followed by an increase in ADAP use. Despite the increase in out-of-pocket costs, both adherence and HIV viral load suppression remained stable.
Conclusions
Medicare Part D was associated with increased out-of-pocket spending, although the increased spending did not appear to compromise ART adherence or HIV viral load suppression. It is possible that increased ADAP use mitigated the increase in out-of-pocket spending, suggesting successful coordination between Medicare Part D and ADAP as well as the vital role of ADAP during insurance transitions.
Keywords: HIV, AIDS Drug Assistance Program, ADAP, antiretroviral therapy, Medicare Part D, Medicaid, Dual Eligible
INTRODUCTION
More than half of US adults with HIV (56%) receive health insurance coverage through Medicare or Medicaid.1 Medicare is a federally administered program that provides health insurance to Americans age 65 and over, as well as persons with permanent disabilities under age 65, who receive Social Security Disability Insurance.2 Medicaid is state-run and provides health insurance to certain categories of low-income persons.3 Ten percent of adults with HIV meet eligibility criteria for both Medicare, primarily through disability criteria rather than age, and Medicaid, through a combination of income and disability criteria, and are enrolled in both programs (“dual eligibles”).4 For dual eligibles, Medicare provides primary coverage while Medicaid absorbs remaining costs and covers services not available through Medicare.3 In addition to Medicaid and Medicare, AIDS Drug Assistance Programs (ADAP) serve as a safety-net program, providing prescription drugs to low-income individuals with limited prescription drug coverage.5 People with HIV rely on these programs for consistent access to antiretroviral therapy (ART), which is crucial to maintaining HIV viral load (VL) suppression.6
For Medicaid enrollees, most states offer a prescription drug benefit with a broad formulary with little to no cost-sharing, including protections that allow enrollees to obtain prescriptions without a co-payment, based on ability to pay.7 Before implementation of Medicare Part D in 2006, Medicare coverage did not include a prescription drug benefit and dual eligibles received prescription drug coverage through Medicaid. Since then, prescription drug coverage has shifted from Medicaid to Medicare and dual eligibles were required to enroll (or be auto-enrolled) in Medicare Part D for prescription drug coverage at implementation.8
Medicare Part D is administered by private prescription drug plans that mandate cost-sharing and vary in the lists of covered drugs and rules for accessing those drugs (utilization management).7,9 Prior to Medicare Part D implementation, policy analysts anticipated that the transition would disrupt ART use for people with HIV in the short-term, and in the long-term due to increased cost-sharing as their coverage through Medicaid was replaced by Medicare Part D.7 Among people with HIV, disruptions in ART can lead to decreased ART adherence and viral suppression, which promote HIV-related morbidity and mortality.10
Among the general population of dual eligibles surveyed in Kansas following Medicare Part D implementation, 20% of dual eligibles reported difficulties filling prescriptions after the transition to Medicare Part D.11 Difficulties filling prescriptions included paying more out-of-pocket for prescription drugs than under Medicaid, needing drugs not covered on their plan’s formulary, and delayed auto-enrollment into Medicare Part D drug plans.11
Only two cross-sectional studies have examined the effects of Medicare Part D on people with HIV, shortly after implementation. One study found that the odds of ART interruption were six times higher among those covered by Medicare Part D compared to those with other or no insurance.12 Increased cost was the primary barrier associated with ART interruption. These findings are supported by reports from HIV providers that the most patients had difficulties accessing their prescription drugs under Medicare Part D.13 Despite reported ART interruption, no studies have examined the effects of Medicare Part D on HIV clinical outcomes, such as viral suppression. Out-of-pocket spending on prescription drugs was of interest because reports of dual eligibles with HIV linked increases in out-of-pocket cost to ART interruption after Medicare Part D,12 even though research on the elderly Medicare population indicated improved medication access after Medicare Part D.14 Further, no studies have examined effects of Medicare part D implementation on ADAP use, despite reported coordination between Medicare Part D and ADAP.15 The effects on ADAP are of interest because, in addition to providing prescription drugs, ADAP can also support people who have certain types of prescription drug coverage, but still face financial barriers to accessing their medications, such as individuals under Medicare Part D.16 The implementation of Medicare Part D also has similarities to the implementation of the Affordable Care Act (ACA) in 2014. Medicare Part D is analogous to the ACA in that individuals with HIV may transition from Medicaid’s prescription drug coverage to private prescription drug coverage, through Part D drug plans under Medicare Part D and under qualified health plans through the ACA’s Health Insurance Exchanges. Medicare Part D and the ACA are also similar in that the private coverage co-functions with ADAP,17,18 mirroring ADAP’s co-functioning with Medicare Part D.15
This study is the first to estimate the effects of Medicare Part D on out-of-pocket prescription drug spending, ADAP use, ART adherence, and viral suppression in dual eligibles with HIV. We used data from the Women’s Interagency HIV Study (WIHS), which include laboratory measures of HIV VL and are collected independent of insurance or pharmacy use, an advantage over clinic or pharmacy claims data.
METHODS
Data source
The WIHS is the largest multisite prospective cohort study of HIV-seropositive and -seronegative women in the United States.19,20 During the time frame for this analysis (2003–2008), the six WIHS study sites were located in the Bronx, NY; Brooklyn, NY; Washington, DC; San Francisco, CA; Los Angeles, CA; and Chicago, IL. Since enrollment began in 1994, the WIHS has collected data on 3,679 HIV-seropositive participants. Biannual study visits include a physical examination, laboratory measurements, and behavioral questionnaires.
Design and study sample
We estimated changes in out-of-pocket prescription drug spending, ADAP use, ART adherence, and viral suppression of dual eligibles after Medicare Part D implementation, compared to a matched sample of Medicaid-only enrollees. We excluded women who missed three consecutive visits between 2003–2008. We restricted the analysis to participants who 1) were HIV-seropositive by January 1, 2003, 2) had at least one visit in both 2005 and 2006, and 3) reported Medicaid-Medicare dual eligibility or Medicaid-only enrollment at Medicare Part D implementation on January 1, 2006. Among 1,634 HIV-seropositive participants, 1,449 (87%) women had least one visit in 2005 and in 2006. Of those, 801 women met the inclusion criteria, of whom 125 were dual eligibles and 676 had Medicaid only.
Measures
Health Insurance Status
The exposure of interest was the transition to Medicare Part D. Participants reporting dual eligibility in 2005 were considered dual eligible at Medicare Part D implementation on January 1, 2006. The control group included participants reporting only Medicaid coverage and no other insurance in 2005. We selected Medicaid-only participants because the two groups had identical prescription drug coverage through Medicaid in the pre-Medicare Part D period.
Outcomes of interest
Several outcomes were considered: 1) self-reported out-of-pocket spending on prescription drugs, 2) self-reported ADAP use, 3) self-reported ART adherence, and 4) HIV viral suppression.
Participants self-reported out-of-pocket prescription drug spending in the last 6 months at every biannual study visit (“none”, “<$25”, “$25–200”, “201–$500”, “>$500”). In 2005, less than a quarter of participants (23%) indicated any out-of-pocket spending, and spending was collapsed to a binary indicator for any out-of-pocket prescription drug spending versus none. We selected a binary measure of out-of-pocket costs because even small cost-sharing increases in have been associated with changes in drug utilization in low-income patients.21,22
We also examined ADAP use, and participants reported whether they used ADAP at each visit. ADAP use was coded as a binary indicator for any use vs. none since the last study visit. A comparison of WIHS data to matched medical record data supports the use of self-reported insurance type as a valid indicator of actual insurance coverage.29
Self-reported ART adherence was assessed as the categorical response to the survey question “Over the past six months, how often did you take your antiretrovirals as prescribed?” where ART adherence was categorized as “100% of the time,” “95–99% of the time,” “75–94% of the time,” and “<75% of the time.” ART adherence was coded as a binary variable, indicating either <100% or 100% adherence since last visit. We also examined an alternative definition of adherence, 95% or greater vs. <95%. VL measurements were taken every six months using the NucliSens assay (Organon Teknika Corp.), which had a lower limit of detection of 80 copies/mL. We defined viral suppression as HIV VL ≤200 copies/mL.24 All outcome measures and covariates were collected every six months throughout the study period.
Statistical Analysis
We explored the relationship between Medicare Part D and outcome variables using a segmented locally weighted smoothed spline (Lowess)25 to visualize trends non-parametrically. We allowed for inflection points at Medicare Part D implementation on January 1, 2006 to visualize discontinuities associated with the transition. A Lowess plot fits a polynomial at each time point using weighted least squares, “smoothing” the outcome levels between data points.
Propensity score matching
We created a propensity score-matched cohort in which we matched dual eligibles with Medicaid-only participants. Under the assumption that the propensity score model was specified correctly, propensity scores should balance covariates between the two groups in the pre-Medicare Part D period, strengthening the assumption that the matched Medicaid-only group represents an appropriate counterfactual for dual eligibles, had that group not transitioned to Medicare Part D.
We used logistic regression to create propensity scores, with dual eligibility as the dependent variable and potential confounders as independent variables. We used a 1:1 nearest neighbor matching approach, without replacement, and dual eligibles were matched with the Medicaid-only participants with the propensity score that was nearest to their own. The covariate balance between dual eligibles and the matched control group was evaluated by comparing standard differences of means and t-test statistics. We included baseline (pre-Medicare Part D) values for the following variables in the logistic regression models to create propensity scores: age at visit, race/ethnicity, education, employment, ADAP use, out-of-pocket prescription drug spending, and HIV VL. Continuous variables (age, VL) were included in the logistic regression as splines26 and categorical variables were dichotomized. We used the psmatch2 program in Stata to perform the 1:1 match.27,21
We estimated the effects of Medicare Part D implementation on dual eligibles with HIV using a difference-in-difference (DiD) approach in a propensity score matched cohort.28 The DiD approach compares the average changes from pre- to post-Medicare Part D in dual eligibles, the group that was affected by the transition, to the average changes during the same time period in participants with Medicaid only, a group unaffected by Medicare Part D. The resulting “difference-in-differences” can be attributed to the policy change, if the assumption of parallel trends is met, the two groups can be balanced on baseline covariates, and there is sufficient overlap in the propensity scores between the groups. Linear regression was used to estimate the change in the proportion of participants experiencing outcomes of interest. Our Medicaid-only control group allowed us to estimate changes in the outcomes of dual eligibles while controlling for temporal trends that are common to both groups (e.g., advances in ART over time).
Finally, we performed sensitivity analyses to test the assumptions inherent in propensity score matching and DiD analyses. We explored the parallel trends assumption using the Lowess plots, tested the balance of baseline covariates, and quantified propensity score overlap of the two groups. Sensitivity analyses included abbreviating pre- and post-Medicare Part D time periods (i.e., restricting to the 2004–2007 and 2005–2006 time periods), examining ART adherence at <95% vs. ≥ 95% adherent, restricting to individuals on ART, and specifying different covariate sets in the propensity score model. All statistical analyses were performed using Stata 13 (StataCorp, College Station, TX).
RESULTS
A total of 801 women were included in this analysis, of which 125 (16%) were dual eligible and 676 (84%) with Medicaid only (Table 1). Median age of dual eligibles was higher than Medicaid-only participants (47; interquartile range [IQR]: 41, 52) vs. 43; IQR: 38, 49, respectively). All participants were under age 65, indicating that dual eligibles became eligible for Medicare coverage through disability criteria, rather than age. Among dual eligibles, 57% were African-American, compared to 68% of Medicaid-only participants. In 2005, 10% of dual eligibles participated in ADAP, compared to the 5% of Medicaid-only participants who participated in ADAP, similar to reports by the National ADAP Monitoring Project.15 A greater proportion of dual eligibles had completed high school or higher compared to Medicaid-only participants (76% vs. 48%); and a lower proportion of dual eligibles reported an annual household income of <$12,000 compared to those with Medicaid only (62% vs. 67%). Finally, a greater proportion of dual eligibles were virally suppressed, compared to Medicaid-only participants (59% vs. 48%), despite similar ART use and ART adherence levels.
Table 1.
Baseline characteristics of Medicaid-Medicare Dual Eligibles and Medicaid-only participants, Women’s Interagency HIV Study (2005)
| Unmatched sample (n=801) | Propensity score-matched sample (n=236) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Dual Eligibles (n=125) |
Medicaid-only (n=676) |
p-valuea | Dual Eligibles (n=118) |
Medicaid-only (n=118) |
p-valuea | |
|
|
|
|||||
| Age in years, median (IQR) | 47 (41, 52) | 43 (38, 49) | 0.005 | 46 (41, 52) | 46 (41, 52) | 0.603 |
| African American, % | 56.5 | 67.9 | 0.014 | 59.0 | 64.4 | 0.394 |
| Hispanic ethnicity, % | 24.2 | 26.6 | 0.575 | 23.9 | 23.7 | 0.971 |
| WIHS site, % | ||||||
| Bronx | 15.2 | 28.7 | 0.002 | 16.1 | 23.7 | 0.144 |
| Brooklyn | 20.0 | 23.5 | 0.391 | 20.3 | 16.9 | 0.506 |
| Washington, DC | 08.0 | 08.6 | 0.831 | 08.5 | 11.9 | 0.391 |
| Los Angeles | 20.0 | 11.0 | 0.005 | 19.5 | 15.3 | 0.393 |
| San Francisco | 24.0 | 15.1 | 0.014 | 22.0 | 17.8 | 0.417 |
| Chicago | 12.8 | 13.2 | 0.912 | 13.6 | 14.4 | 0.852 |
| ADAP | 10.4 | 05.1 | 0.019 | 07.7 | 07.6 | 0.985 |
| Any out-of-pocket prescription spending, % | 22.8 | 12.9 | 0.004 | 21.6 | 14.5 | 0.165 |
| 100% ART adherentb | 51.2 | 43.2 | 0.127 | 51.2 | 48.4 | 0.719 |
| CES-D, median (IQR) | 14 (3.5, 28.5) | 15 (6, 25) | 0.844 | 14 (3, 27) | 15 (4, 27) | 0.698 |
| Household income <$12,000/year, % | 62.1 | 67.1 | 0.278 | 62.2 | 60.9 | 0.849 |
| Graduated high school, % | 76.4 | 48.1 | <0.0001 | 25.5 | 27.0 | 0.792 |
| Employed, % | 12.9 | 18.6 | 0.129 | 12.1 | 10.0 | 0.558 |
| CD4 cell count, median (IQR) | 466 (312, 643) | 416 (249, 622) | 0.265 | 484 (324, 658) | 476 (316, 728) | 0.396 |
| Virally suppressedc, % | 59.3 | 48.0 | 0.021 | 59.6 | 64.7 | 0.447 |
Abbreviations: ADAP, AIDS Drug Assistance Programs; ART, antiretroviral therapy; CES-D, Center for Epidemiologic Studies Depression Scale; IQR, interquartile range; HIV, Human Immunodeficiency Virus; WIHS, Women’s Interagency HIV Study
Statistical significance tested using t tests
Proportions calculated within subset of dual eligibles (n=103) and Medicaid-only participants (n=461) on ART
Viral suppression corresponds to a VL measurement of <200 copies/mL
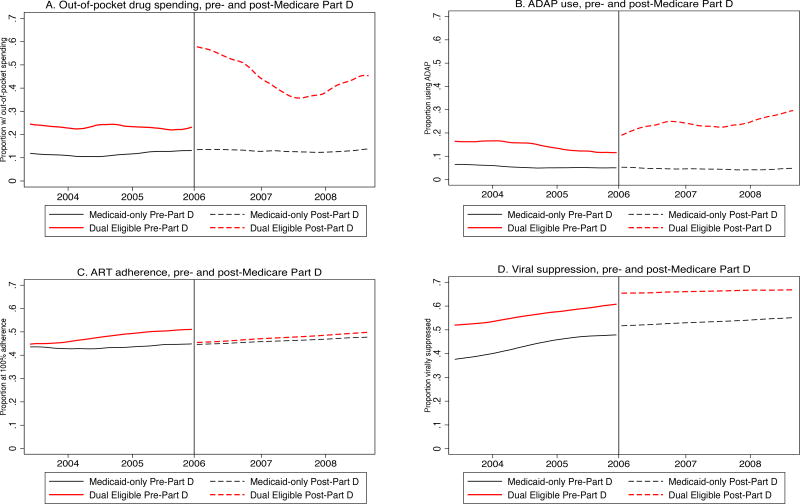
Following Medicare Part D implementation, Lowess plots showed a sharp increase in out-of-pocket prescription drug spending in 2006 (Figure 1A). While reported out-of-pocket spending decreased over the following two years, dual eligibles’ out-of-pocket spending did not return to pre-Medicare Part D levels. Lowess plots showed a more gradual increase in ADAP use among dual eligibles (Figure 1B).
Figure 1.
A–D Change in Proportion of Outcome of Interest, by Insurance Type, Time Period in the Women’s Interagency HIV Study (WIHS)
Abbreviations: ADAP, AIDS Drug Assistance Program; ART, Antiretroviral Therapy
Lowess plots of ART adherence showed no inflection points for either group. viral suppression appeared to be increasing over time in both groups, possibly corresponding to advances in ART, with no discontinuity following Medicare Part D implementation (Figures 1C–D). Lowess plots also indicated that the parallel trend assumption held for all outcomes of interest during the pre-Medicare Part D time period, strengthening the validity of the DiD analyses. For all Lowess plots, at least 93% of the analytic sample contributed data to each time point. In the analytic sample, the proportion of missing values was below 6% at Medicare Part D implementation for all outcome measures and covariates, and below 7% throughout the full study period, with the exception of out-of-pocket prescription drug spending, which rose to 13% in 2008.
The set of variables used in the propensity score matching resulted in a covariate balance between the two groups on sociodemographics, medication use and related spending, and health status (Table 1) In the propensity-score matched DiD analyses, dual eligibles showed increases in out-of-pocket spending on prescription drugs, with 23% reporting any out-of-pocket spending for prescription drugs in the pre-Medicare Part D period to 41% in the post-Medicare Part D time period (Table 2). Adjusting for any temporal trends by subtracting the change in the matched control group, the DiD estimate attributed to the transition to Medicare Part D was an average 20% change (95% CI: 12%-27%) in proportion of dual eligibles reporting out-of-pocket spending. ADAP use increased by 10% among dual eligibles following Medicare Part D implementation (95% CI: 3%-18%).
Table 2.
Difference-in-Differences Estimates – Average Proportion Change in Pre- and Post-Medicare Part D Time Period, by Insurance Type, Women’s Interagency HIV Study 2003–2008
| % with OOP spending | % Using ADAP | % ART adherenta | % Virally suppressed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| % | SE | p-value | % | SE | p-value | % | SE | p-value | % | SE | p-value | |
|
|
|
|
|
|||||||||
| Pre-Medicare Part D (2003–2005) | ||||||||||||
| Medicaid-only | 0.23 | 0.13 | 0.39 | −0.50 | ||||||||
| Dual eligible | 0.24 | 0.14 | 0.47 | −0.55 | ||||||||
| Difference | 0.01 | 0.04 | 0.859 | 0.01 | 0.04 | 0.716 | 0.08 | 0.05 | 0.155 | −0.05 | 0.05 | 0.361 |
| Post-Medicare Part D (2006–2008) | ||||||||||||
| Medicaid-only | 0.21 | 0.10 | 0.44 | −0.62 | ||||||||
| Dual eligible | 0.41 | 0.22 | 0.48 | −0.66 | ||||||||
| Difference | 0.20 | 0.04 | <0.001 | 0.12 | 0.04 | 0.004 | 0.04 | 0.05 | 0.440 | −0.05 | 0.05 | 0.337 |
| Difference-in-Differences | 0.20 | 0.04 | <0.001 | 0.10 | 0.04 | 0.007 | −0.04 | 0.05 | 0.440 | −0.001 | 0.05 | 0.987 |
Abbreviations: ADAP, AIDS Drug Assistance Program; ART, Antiretroviral Therapy; OOP, out-of-pocket; VL, viral load; SE, standard error
Proportion reporting 100% ART adherence
Levels of self-reported ART adherence were comparable in dual eligibles and Medicaid-only enrollees in the pre-Medicare Part D time period (47% vs. 39%) and in the post-Medicare Part D time period (48% vs. 44%), and DiD estimation did not attribute a significant change to the transition. Sensitivity analyses using other adherence cut-points did not alter these results. Similarly, DiD estimation did not attribute a significant change in the proportion of dual eligibles who were virally suppressed, after adjusting for temporal trends. Finally, the number of dual eligibles could not support sub-analyses by ADAP use and restriction to participants on ART did not substantially change the direction or magnitude of any of the outcome variables.
DISCUSSION
This is the first study to examine the effects of Medicare Part D implementation on out-of-pocket prescription medication costs, ART adherence, viral suppression, and ADAP use among HIV-seropositive women enrolled in Medicaid and Medicare (dual eligibles). As anticipated, the proportion of dual eligibles reporting out-of-pocket spending for prescription drugs increased following Medicare Part D implementation. Despite this increase, ART adherence and viral suppression remained stable after the transition. The proportion of dual eligibles using ADAP also increased after implementation, though the increase was more gradual. Taken together, these results suggest that while the transition to Medicare Part D was associated with increased self-reported out-of-pocket costs and ADAP use, ART adherence and viral suppression remained stable.
Cost-Sharing and Out-of-Pocket Prescription Drug Spending
Our findings differed from previous research that reported no change in dual eligibles’ out-of-pocket prescription drug costs in either the transition or the stable period following Medicare Part D implementation.30 However, those study results were based on a sample of dual eligibles who were >65 years of age, whose health needs differ from non-elderly populations with HIV. Our findings are supported by the one previous study of HIV-seropositive individuals, in which 60% of those enrolled in a Medicare Part D prescription drug plans reported increased out-of-pocket prescription drug expenditures shortly after implementation.12
ADAP Use and Medicare Part D
Lowess plots indicate an increase in ADAP use following the increase in out-of-pocket prescription drug spending. These findings are supported by reports of coordinated coverage of dual eligibles through Medicare Part D and ADAP.15 Despite the rise in ADAP use and the financial advantages of using ADAP in combination with Medicare Part D, only 22% of dual eligibles in this study reported ADAP use and 41% still reported out-of-pocket prescription drug spending in 2008.
ART Adherence and Viral Suppression
Given reports of ART interruption and increased out-of-pocket prescription drug costs shortly after Medicare Part D implementation,12,13 we hypothesized that increased out-of-pocket prescription drug spending would lead to decreased ART adherence, and consequently, decreased viral suppression. However, dual eligibles’ ART adherence remained stable. There are several explanations for stable ART adherence. First, increased enrollment in ADAP may have mitigated the effects of prescription drug spending by absorbing out-of-pocket-costs, resulting in stable ART adherence. Second, despite increases in the proportion of dual eligibles with any out-of-pocket prescription drug spending, the bulk of participants reported low out-of-pocket spending. For persons with out-of-pocket costs, 54% of participants reported out-of-pocket costs ranging from $1-$25, and 42% had costs ranging from $26-$200 in the prior six months. Even though two-thirds (66%) of participants reported a household income <$12,000 per year, costs may not have been high enough to lead to cost-related ART non-adherence. Finally, we also considered that the burden of out-of-pocket spending may have led to reduced spending on other essential needs (e.g., food, child care)31 or that WIHS participants may have been more conscientious about adherence due to their long-term study involvement.32
Similarly, we found no evidence of changes in viral suppression in dual eligibles associated with Medicare Part D. Though the proportion of dual eligibles who were virally suppressed increased between the pre- and post-Medicare Part D time periods, a similar trend was observed in the Medicaid-only group, indicating that both groups benefited from improvements in ART. These results suggest that stable viral suppression may have resulted from increased ADAP use, rather than improved medication access through Medicare Part D. This interpretation is supported by studies in which ADAP was associated with an increased ART use33 and increased likelihood of viral suppression.29
Limitations
Out-of-pocket costs, ART adherence, and ADAP use are self-reported in the WIHS over a period of six months, which may have led to misclassification. Our study was also limited to dual-eligible women with HIV who participate in a longitudinal cohort study, and results may not be generalizable to all dual eligibles with HIV. Even though the WIHS is the largest observational cohort of women with HIV, the six WIHS sites represent a limited number of US states, and the transition from state-run Medicaid programs to Medicare Part D in this study may not be generalizable to all US states. Propensity scores can only balance groups on measured covariates, and, as in all observational studies, unmeasured covariates may confound our results. Finally, dual eligibles make up a small proportion of people with HIV and, consequently, this study’s sample size was relatively small. However, even though dual eligibles represent a small proportion of Medicare enrollees and our sample size was limited, their unique healthcare utilization and co-morbidity patterns make their study significant.
Despite these limitations, this study has several unique advantages: study visits occur at six-month intervals and are independent of insurance status or prescription fill behavior. Further, study visits are standardized, and, as such, the WIHS cohort is consistently characterized over time. This study has an additional advantage that it assessed viral suppression, a key indicator of effective HIV treatment. Finally, this study’s longitudinal design allows analysis of dual eligibles over time, for several years before and after Medicare Part D implementation.
CONCLUSIONS
Prior studies showed improved medication access following Medicare Part D implementation in many Medicare enrollees. However, dual eligible women with HIV, an understudied group, did not reflect those improvements in medication access or reduced out-of-pocket prescription drug costs seen in other Medicare enrollees. Our results underscore the importance of considering ADAP’s role in maintaining medication access and viral suppression during federally mandated insurance coverage transitions. While ADAP is essential in providing HIV medications to the uninsured, it may also benefit dual eligibles with HIV.
This study has implications beyond Medicare Part D and dual eligibles with HIV. Medicare Part D’s privatized, market-based prescription drug plans are analogous to the privatized, market-based coverage that people with HIV encounter through the ACA’s Health Insurance Exchanges. An additional similarity is that the ACA allows ADAP to provide similar benefits for people with HIV as ADAP provided for dual eligibles under Medicare Part D. These findings suggest that safety-net programs such as ADAP may play a role in ensuring smooth insurance coverage transitions, an important consideration as people with HIV transition to private prescription drug coverage under the ACA.
Acknowledgments
Funding: This research was partially supported by a National Research Service Award Pre-Doctoral/Post-Doctoral Traineeship from the Agency for HealthCare Research and Quality sponsored by The Cecil G. Sheps Center for Health Services Research, The University of North Carolina at Chapel Hill, Grant No. T32-HS000032, and by an Agency for HealthCare Research and Quality Individual Award for Postdoctoral Fellows (F32) National Research Service Award (F32-HS024858).
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), and P30-AI-050410 (UNC CFAR).
Footnotes
Conflict of Interest: NA
References
- 1.Kaiser Family Foundation. Medicaid and HIV: A National Analysis. 2011. [Google Scholar]
- 2.Kaiser Family Foundation. Medicaid’s Role for Dual-Eligible Beneficiaries [Internet] 2013 Available from: http://kff.org/medicaid/issue-brief/medicaids-role-for-dual-eligible-beneficiaries/
- 3.Young K, Garfield R, Musumeci M, Clemans-Cope L, Lawton E. Medicaid’s Role for Dual Eligible Beneficiaries. Kaiser Family Foundation; 2013. [Google Scholar]
- 4.Committee on HIV Screening and Access to Care. HIV Screening and Access to Care: Exploring the Impact of Policies on Access to and Provision of HIV Care. Institute of Medicine, The National Academies Press; 2011. [PubMed] [Google Scholar]
- 5.The Henry J. Kaiser Family Foundation. National ADAP Monitoring Project 2007 Annual Report. 2007. [Google Scholar]
- 6.Reif S, Whetten K, Lowe K, Ostermann J. Association of Unmet Needs for Support Services with Medication Use and Adherence among HIV-Infected Individuals in the Southeastern United States. AIDS Care. 2006;18(4):277–83. doi: 10.1080/09540120500161868. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser Family Foundation. The Role of Part D for People with HIV/AIDS: Coverage and Cost of Antiretrovirals under Medicare Drug Plans. Menlo Park: Henry J. Kaiser Family Foundation; 2006. [Google Scholar]
- 8.United States Government Accountability Office. Challenges in Enrolling New Beneficiaries Highlights Challenges in Enrolling New Dual-Eligible. 2007. May, [Google Scholar]
- 9.Smith VK. The Transition of Dual Eligibles to Medicare Part D Prescription Drug Coverage: State Actions During Implementation. Henry J. Kaiser Family Foundation; 2006. [Google Scholar]
- 10.Holkmann Olsen C, Mocroft A, Kirk O, Vella S, Blaxhult A, Clumeck N, et al. Interruption of Combination Antiretroviral Therapy and Risk of Clinical Disease Progression to AIDS or Death. HIV Med. 2007;8(2):96–104. doi: 10.1111/j.1468-1293.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 11.Hall JP, Kurth NK, Moore JM. Transition to Medicare Part D: An Early Snapshot of Barriers Experienced by Younger Dual Eligibles with Disabilities. 2007. [PubMed] [Google Scholar]
- 12.Das-Douglas M, Riley ED, Ragland K, Guzman D, Clark R, Kushel MB, et al. Implementation of the medicare part D prescription drug benefit is associated with antiretroviral therapy interruptions. AIDS Behav. 2009;13(1):1–9. doi: 10.1007/s10461-008-9401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.HIV Medicine Association. HIV Medical Provider Medicare Part D Survey [Internet] Available from: http://www.hivdent.org/_medicare_/PDF/HIVmedicalprovidersurveyreport.pdf.
- 14.Neuman P, Strollo MK, Guterman S, Rogers WH, Li A, Rodday AMC, et al. Medicare prescription drug benefit progress report: Findings from A 2006 National Survey of Seniors. Health Aff [Internet] 2007;26(5) doi: 10.1377/hlthaff.26.5.w630. Available from: http://dx.doi.org/10.1377/hlthaff.26.5.w630. [DOI] [PubMed] [Google Scholar]
- 15.National ADAP Monitoring Project, Annual Report. 2006 Mar; Available from: https://kaiserfamilyfoundation.files.wordpress.com/2013/01/7464.pdf.
- 16.The Henry J. Kaiser Family Foundation. National ADAP Monitoring Project 2006 Annual Report. 2006. [Google Scholar]
- 17.McManus KA, Rodney RC, Rhodes A, Bailey S, Dillingham R. Affordable Care Act Qualified Health Plan Enrollment for AIDS Drug Assistance Program Clients: Virginia’s Experience and Best Practices. AIDS Res Hum Retroviruses. 2016 Sep;32(9):885–91. doi: 10.1089/aid.2016.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Alliance of State & Territorial AIDS Directors (NASTAD) National ADAP Monitoring Project: 2015 Annual Report [Internet] 2015 Available from: http://www.nastad.org/sites/default/files/NASTAD-ADAP-Monitoring-Project-Annual-Report-May-2015.pdf.
- 19.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish La, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group [Internet]. Vol. 9, Epidemiology (Cambridge, Mass.) 1998:117–25. Available from: http://dx.doi.org/10.1097/00001648-199803000-00004. [PubMed]
- 20.Hessol NA, Weber KM, Holman S, Robison E, Goparaju L, Alden CB, et al. Retention and Attendance of Women Enrolled in a Large Prospective Study of HIV-1 in the United States. J Womens Health. 2009;18(10):1627–37. doi: 10.1089/jwh.2008.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeder CE, Nelson AA. The differential impact of copayment on drug use in a Medicaid population. Inquiry. 1985 Winter;22(4):396–403. [PubMed] [Google Scholar]
- 22.Tamblyn R, Laprise R, Hanley JA, Abrahamowicz M, Scott S, Mayo N, et al. Adverse events associated with prescription drug cost-sharing among poor and elderly persons. JAMA. 2001;285(4):421–9. doi: 10.1001/jama.285.4.421. [DOI] [PubMed] [Google Scholar]
- 23.Reed M, Brand R, Newhouse JP, Selby JV, Hsu J. Coping with prescription drug cost sharing: knowledge, adherence, and financial burden. Health Serv Res. 2008 Apr;43(2):785–97. doi: 10.1111/j.1475-6773.2007.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2009. pp. 1–161. [Google Scholar]
- 25.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. J Am Stat Assoc. 1979;74(368):829–36. [Google Scholar]
- 26.Stata Corp. mkspline — Linear and restricted cubic spline construction. Stata J [Internet] Available from: http://www.stata.com/manuals13/rmkspline.pdf.
- 27.Leuven E, Sianesi B. PSMATCH2: Stata module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. Statistical Software Components; 2014. [Google Scholar]
- 28.Stuart Ea, Huskamp Ha, Duckworth K, Simmons J, Song Z, Chernew ME, et al. Using propensity scores in difference-in-differences models to estimate the effects of a policy change. Health Serv Outcomes Res Methodol. 2014:166–82. doi: 10.1007/s10742-014-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludema C, Cole SR, Eron JJ, Jr, Edmonds A, Holmes GM, Anastos K, et al. Impact of Health Insurance, ADAP, and Income on HIV Viral Suppression Among US Women in the Women’s Interagency HIV Study, 2006–2009. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016 Nov 1;73(3):307. doi: 10.1097/QAI.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin W, Basu A, Zhang JX, Rabbani A, Meltzer DO, Alexander GC. The Effect of the Medicare Part D Prescription Benefit on Drug Utilization and Expenditures. Ann Intern Med. 2008;148(3):169–77. doi: 10.7326/0003-4819-148-3-200802050-00200. [DOI] [PubMed] [Google Scholar]
- 31.Heisler M, Wagner TH, Piette JD. Patient strategies to cope with high prescription medication costs: Who is cutting back on necessities, increasing debt, or underusing medications? J Behav Med. 2005;28(1):43–51. doi: 10.1007/s10865-005-2562-z. [DOI] [PubMed] [Google Scholar]
- 32.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. doi: 10.1186/1471-2288-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi T, Cocohoba J, Cohen M, Anastos K, DeHovitz JA, Kono N, et al. The Impact of the AIDS Drug Assistance Program (ADAP) on Use of Highly Active Antiretroviral and Antihypertensive Therapy among HIV-Infected Women. J Acquir Immune Defic Syndr. 2011;56(3):253. doi: 10.1097/QAI.0b013e31820a9d04. [DOI] [PMC free article] [PubMed] [Google Scholar]