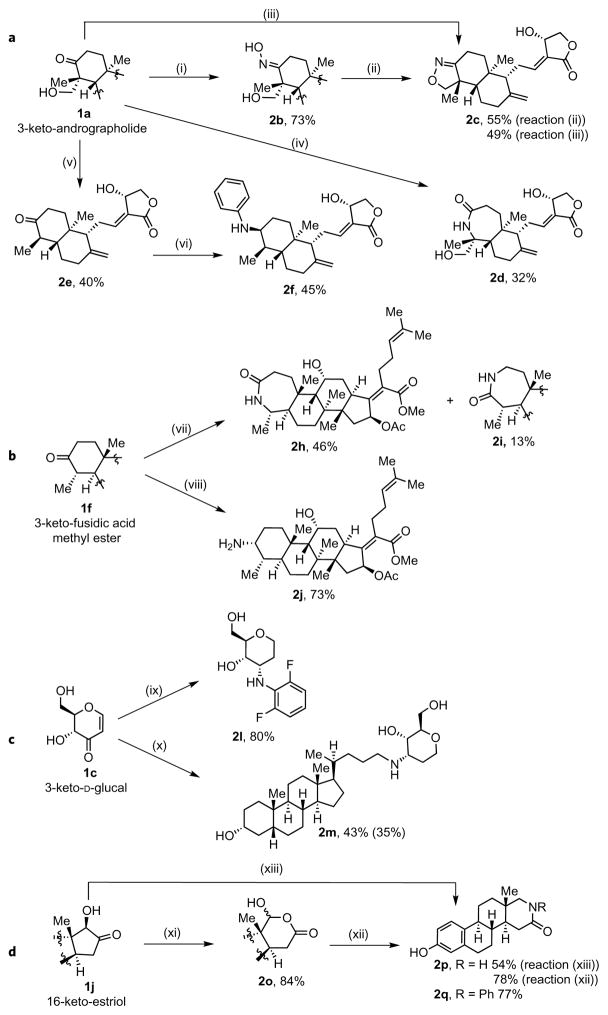
Figure 4. Oxidation and amination of structurally complex polyol natural products.
a, The incorporation of nitrogen-based functional groups into andrographolide occurs by the formation of oxime derivatives and catalytic reductive amination. (i) NH2OH–HCl, pyridine, 40 °C, 4 h; (ii) TsCl, NEt3, THF, 40 °C, 2 d; (iii) NH2OSO3H, 1:1 0.01% TFA:TFE, 50 °C, 24 h; (iv) NH2OSO3H, 1:1 2.5% NaHCO3(aq):TFE, 50 °C, 40 min, then 80 °C, 60 min; (v) 2SmI2, methyl acrylate, 4:1 THF:TFE, 65 °C, 25 min; (vi) 3% Ir-1, C8H9NH2, HCO2H, MeOH, 65 °C. b, The incorporation of nitrogen-based functional groups into fusidic acid methyl ester is achieved by a direct conversion of the ketone formed by oxidation into the corresponding lactam and by catalytic and diastereoselective reductive amination of this ketone to the free amine. (vii) NH2OSO3H, 1:1 H2O:hexafluoroisopropanol, 50 °C, 20 min, then 80 °C, 30 min, then 1:1 H2O:Me2CO, 80 °C, 10 min; (viii) 2.5% Ir-1, NH4O2CH, MeOH, 65 °C, 4 h. c, The incorporation of nitrogen-based functional groups into D-glucal is achieved by catalytic and diastereoselective reductive amination. The conjugation of two natural products was achieved by this approach. (ix) 2.5% Ir-1, C5H4F2NH3O2CH, MeOH, 65 °C, 10 h; (x) Ir-1, lithocholic amine, HCO2H, MeOH, 65 °C, 20 h. d, The incorporation of nitrogen-based functional groups into oestriol is achieved by a one-step catalytic conversion of the hydroxyketone to give the corresponding lactam or by the combination of Baeyer–Villiger oxidation and reductive amination to yield N-substituted lactams. (xi) 3% Pt-1, H2O2(aq), THF, r.t., 2 d or 3% Pt-1, H2O2(aq), THF, 45 °C, 6 h (77% yield); (xii) R = H, 2.5% Ir-1, NH4O2CH, MeOH, 65 °C, 4 h; R = Ph, 2.5% Ir-1, PhNH3O2CH, MeOH, 12 h; (xiii) 2.5% Ir-1, NH4O2CH, MeOH, 65 °C, 4 h.