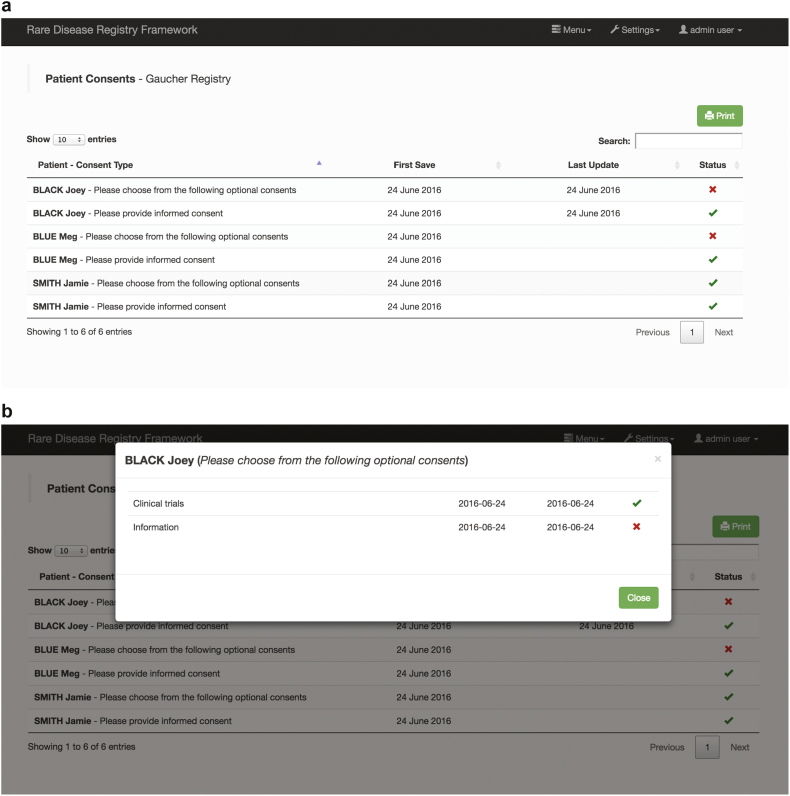
Fig. 2.
Configurable and customisable consent.
a) Consent is now easily customised, with validation and applicability rules available. Time-stamping of when consent is provided in a searchable table of consent sections. This assists Data Curators to identify patients who have not provided informed consent; b) Further information on individual consent questions can be obtained by clicking on the patient name.