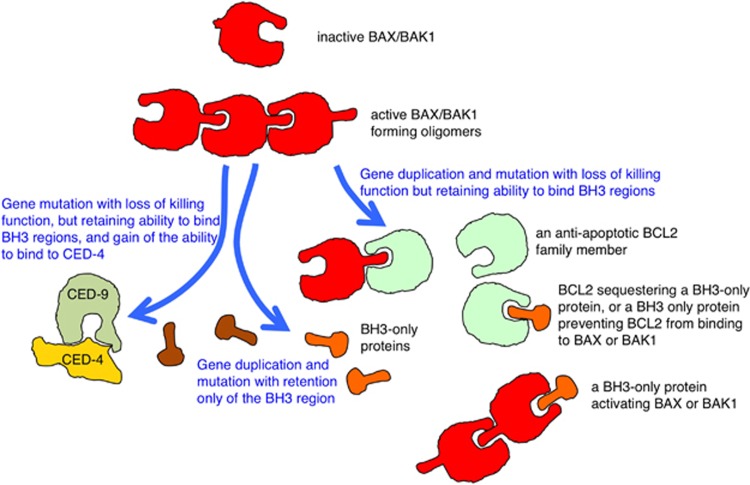
Figure 3.
Speculative evolution of the BCL2 protein subfamilies. The first members were like pro-apoptotic BAX and BAK1, and had three functions: exposing a BH3 domain; binding a BH3 domain of another protein; and forming oligomers that could act as channels allowing proteins to cross membranes. Anti-apoptotic family members (such as BCL2 itself) arose by gene duplication from a BAX/BAK1-like ancestor, and mutations that caused the protein to lose its ability to expose its BH3 domain in a way that higher-order multimers could form, whilst retaining its ability to bind to the BH3 domains of other proteins, such as BAX and BAK1. In this way, anti-apoptotic BCL2 family members act as dominant negative versions of BAX and BAK1. BH3-only family members arose as proteins that could bind to anti-apoptotic BCL2 family members to unleash BAX or BAK1, or could bind to BAX or BAK1 to activate them directly, in both ways triggering formation of multimers. Cartoons are diagrammatic only