Figure 1.
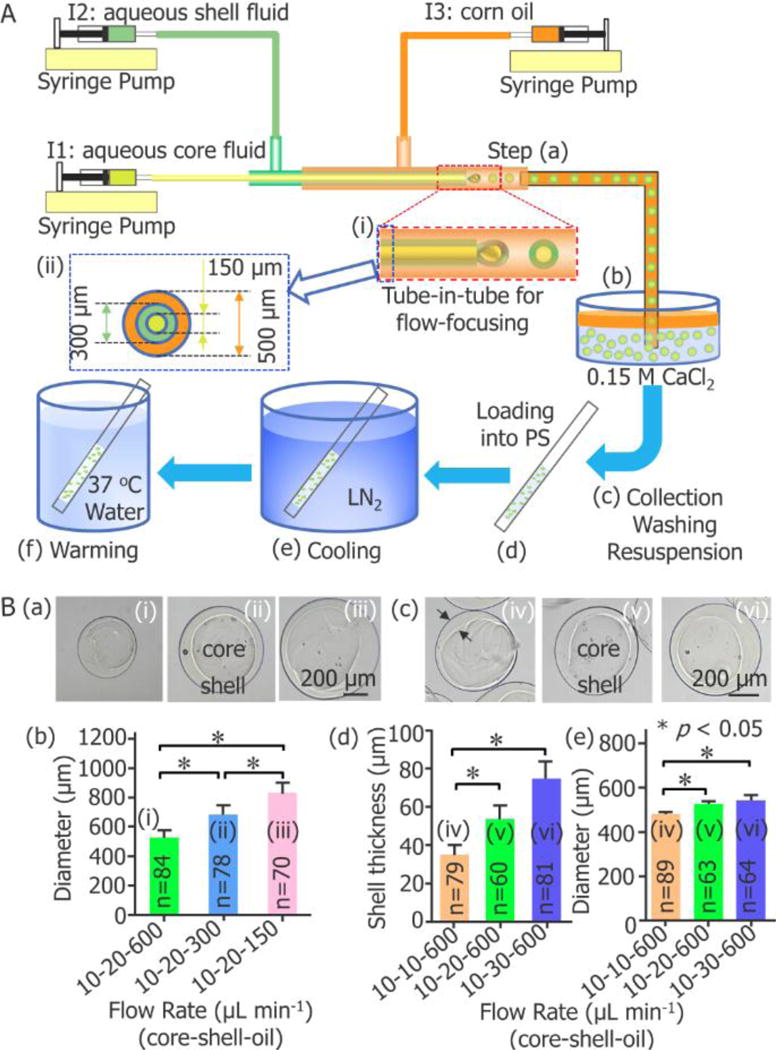
Capillary microfluidic encapsulation of stem cells in core-shell microcapsules for vitreous cryopreservation. (A) A schematic illustration of the major steps: (a) cells are encapsulated in droplets using a double emulsion flow-focusing tube-in-tube capillary microfluidic device, (b) the droplets are immersed into aqueous CaCl2 solution for crosslinking into microcapsules with a calcium alginate hydrogel shell, (c) the microcapsules are collected, washed, and resuspended in 0.9% NaCl solution, (d) after incubated in a cryoprotective agent (CPA) solution, the microcapsules are loaded into a plastic straw (PS), (e) after sealed at the distal end using wax, the PS is plunged into liquid nitrogen (LN2) for cooling, and (f) the PS is plunged into 37 °C waterbath for warming the sample. I1: ice-cold cell suspension with 1% (wt) sodium carboxymethylcellulose, 1% (wt) sodium alginate, and 0.5 mol L−1 trehalose in 0.25 mmol L−1 D-Mannitol. I2: 2% (wt) sodium alginate solution. I3: corn oil. (B) Fabrication of core-shell microcapsules with controllable outer diameter and shell thickness. (a) and (b) show the dependence of the diameter of the microcapsules on the flow rate of the continuous oil phase (n = 70–84, * p < 0.05). (c) - (e) show the dependence of both the shell thickness and diameter of the microcapsules on the flow rate of the aqueous shell fluid (n = 60–89, * p < 0.05).
