Fig. 1.
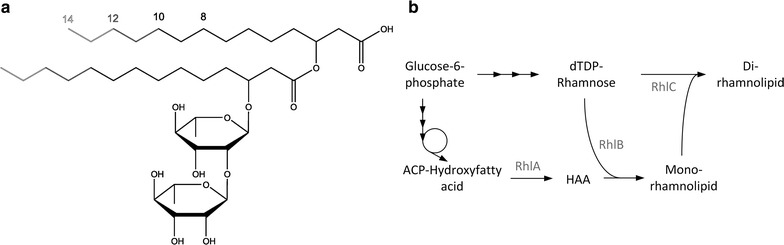
a Structure of rhamnolipids. The upper part of the molecule is formed by the hydrophobic moiety, the hydroxyalkanoyloxy alkanoate (HAA). The chain lengths of the β-hydroxy-fatty acids in this dimer can vary. One or two rhamnose molecules are bound by a glycosidic bond. The sugar molecules are the hydrophilic moiety of the molecule. Molecules with one rhamnose are called mono-rhamnolipids, while the here depicted molecule with two rhamnoses is a di-rhamnolipid. b Biosynthesis pathways of rhamnolipids. Based on glucose two pathways are required for rhamnolipid synthesis. In the lower part, activated β-hydroxy-fatty acids are formed via fatty acid de novo synthesis, which are fused by the enzyme RhlA. In the upper part, activated rhamnose is synthesized and subsequently coupled to the β-hydroxy-fatty acid dimer by the rhamnosyltransferase I (RhlB). The rhamnosyltransferase II (RhlC) finally adds another sugar molecule to yield a di-rhamnolipid. Enzyme names are printed in grey
