Abstract
The St. George’s Respiratory Questionnaire (SGRQ) and chronic obstructive pulmonary disease (COPD) assessment test (CAT) are the measures used to assess health status. This study aims to examine the responsiveness of these tools by severity of dyspnoea category in patients with COPD. Forty-nine COPD patients who underwent a 12-week pulmonary rehabilitation (PR) programme were assessed at baseline, 12 weeks and at 28-week follow-up. Patients were categorized into two groups by severity of dyspnoea category (i.e. mild to moderate (modified Medical Research Council (mMRC) 1–2) and severe to very severe (mMRC 3–4)) using the mMRC dyspnoea scale. Effect size (ES) was computed as estimates of responsiveness. The SGRQ demonstrated greater responsiveness by total sample (SGRQ, ES = 0.87; CAT, ES = 0.75) and for the mMRC 3–4 category (SGRQ, ES = 0.91; CAT, ES = 0.76) on completion of PR. At 28-week follow-up, overall comparable responsiveness of the CAT and SGRQ was identified by total sample (SGRQ, ES = 0.75; CAT, ES = 0.74) and by severity of dyspnoea category. The symptom, impact and activity domains of the SGRQ showed good responsiveness, with greater ESs obtained overall for the mMRC 3–4 category. On completion of PR, the SGRQ demonstrates a greater responsiveness with COPD patients, especially in relation to the mMRC 3–4 category, while both the CAT and SGRQ show comparable responsiveness on follow-up.
Keywords: Health-related quality of life, COPD, pulmonary rehabilitation, exercise, responsiveness
Introduction
Chronic obstructive pulmonary disease (COPD) is a devastating respiratory condition and a major cause of morbidity and mortality.1,2 Taking into account the fact that a complete cure is not possible, patients undergo intensive pulmonary rehabilitation (PR), an evidence-based intervention which is identified as one of the major interventions for the management of COPD.3 One of the major aims of PR is that of ameliorating the health-related quality of life of individuals with chronic respiratory impairment.2,4–7
Two questionnaires commonly used within the care of COPD patients are the St. George’s Respiratory Questionnaire (SGRQ)8,9 and COPD assessment test (CAT).10,11 Clinically, the SGRQ has been demonstrated to be an important tool to quantify the impact of COPD on symptoms, functional measures and general well-being of the patient,12,13 as well as in evaluating the effectiveness of healthcare services.14 Despite this, the SGRQ is considered to be long, complex and time-consuming to complete.15 The CAT was developed in order to have a short and easy tool which could be administered in the clinical setting.11 It is a validated tool measuring and quantifying the impact or burden of COPD on the individual.16 Both tests have been compared individually for changes in health status following PR,17 with evidence for responsiveness7,18,19 identified for both tools. The responsiveness of a tool is considered an important property when choosing a questionnaire, as it detects the capacity of the tool to detect change over time.20,21 When assessing changes in health status before and after an intervention, responsiveness is an expression of the ability of that tool to detect any differences between the questionnaire scores, in a manner which reflects the change that would have taken place.22,23
The need to identify a tool having a good responsiveness is of importance for COPD patients, considering that between 8.3% and 49.6% of those referred for PR do not attend and between 9.7% and 31.8% fail to complete the programme.24 Obstacles cited by COPD patients in relation to non-completion of PR include severity of breathlessness, transportation difficulties, depression, programme perceived as too long and lack of perceived benefit.24,25 Consequently, there is a need to identify a tool that has a good responsiveness in relation to health status measures, such as the severity of breathlessness, which has been identified as a significant predictor of non-completion of PR programmes. This would also facilitate the introduction of individualized PR programmes to enhance adherence, as recommended by the UK National Audit on PR.26
This article reports a study that aims to contribute to the existing literature, by examining the responsiveness of the CAT and SGRQ, on stable COPD patients categorized by dyspnoea severity level, following a 12-week PR programme and at 28-week follow-up.
Methods
A quantitative, longitudinal study was conducted. Data were collected at baseline, on completion of PR at 12 weeks and at 28-week follow-up.
Study design and participants
Seventy-five patients (59 males and 16 females) with a confirmed diagnosis of COPD were referred from the medical wards and respiratory outpatient clinic of the local general hospital. The definition of COPD adopted for this study was provided by the American Thoracic Society/European Respiratory Society Guidelines.27 Patients had a self-reported smoking history, clinical signs and symptoms together with spirometry readings which were consistent with COPD and exertional dyspnoea (modified Medical Research Council (mMRC) dyspnoea score – grade 1 or above). These participants were all found to be medically stable by the respiratory physicians and free from exacerbations in the 3 months preceding enrolment into PR. Inclusion criteria to the rehabilitation programme included oxygen saturations of >92% at rest, stable cardiovascular system and no neurological or orthopaedic problems which could interfere with rehabilitation. Additionally, participants who required modifications to their drug therapy due to exacerbations were excluded from the study.
Rehabilitation programme
The rehabilitation programme consisted of twice weekly 2-hour sessions, over 12 weeks. The exercise session consisted of a warm-up period, treadmill walking, with the initial speed devised from the distance obtained from the 6-minute walk test and gradually increased throughout the weeks, step-climbing, arm ergometry, cycling using a stationary bike and also upper and lower limb strengthening exercises using weights. Inspiratory muscle training was also carried out using the Respironics inspiratory muscle trainer (IMT) Threshold trainer® for 15 minutes during the class. All participants were instructed to carry the IMT at home for 30 minutes, 5 days per week. A home exercise programme was also provided to the patients. Educational sessions were conducted on aspects of COPD care and self-management by medical doctors, psychologists, physiotherapists, dieticians and respiratory nurses. These sessions were monitored by a home diary system provided to each participant at the start of the programme.
Questionnaires
Patients were assessed 2 weeks before enrolling into the programme (i.e. at baseline), on completion of the 12-week PR programme and during the follow-up phase at 28 weeks. Health status measures were obtained using the SGRQ8 and CAT.10
The SGRQ8 consists of 50 items, within three sections representing the symptom, activity and impact domains. The scores range from 0 to l00 for the three subscales with a summary total score. Higher scores indicate worse health status; 0 indicates no impairment and 100 indicates maximal impairment.9 The SGRQ has been shown to have an adequate inter-rater reliability and reproducibility as well as the ability to show responsiveness to quantify change over time.9
The CAT11 assesses several aspects affecting COPD patients ranging from symptoms, health status and well-being. Each question presents with a statement with a rating ranging from the best (score of 0) to the worst (score of 5) rating for that statement.10 The scores for each of the eight items are then added up to give one final score (with a minimum of 0 and a maximum score of 40). The higher the value of the total score, the worse the health status of the individual. The internal consistency (Cronbach α = 0.88) was high for total score, as well as having good reproducibility (interclass correlations [ICC] = 0.80).
The mMRC dyspnoea score is obtained from a questionnaire consisting of five statements, which provide a measure of perceived breathlessness. For statistical purposes, participants were divided by dyspnoea scale category with the ‘mild to moderate category’ consisting of patients who obtained a score of ‘1’ or ‘2’ on the mMRC and the ‘severe to very severe category’ for those scoring a ‘3’ or ‘4’. Fifteen participants had an mMRC dyspnoea score of 1; 14 had an mMRC score of 2 (n = 14) and 10 individuals, respectively, had an mMRC score of 3 and 4 (n = 10).
Ethical considerations
An information letter was provided to participants with details regarding the nature of the study. Participants were also informed that they could withdraw from the research study at any point in time. Informed consent was requested as an indication of their voluntary participation in the rehabilitation programme. All data collected from the participants were coded to ensure patient confidentiality. Ethical approval was obtained from the relevant institutional research ethics committee (191/2011).
Statistical analysis
Statistical analysis was conducted using the Statistical Package for Social Science (SPSS) software version 23. Baseline characteristics and exercise data are presented as mean ± standard deviation (SD). In order to examine changes in scores obtained at baseline, with those obtained on completion of the programme at 12 weeks and then on follow-up at 28 weeks, the paired samples t-test was used. This test is appropriate when the samples comprise matched pairs, typically found in ‘before–after’ studies. Data were checked for normality using the Shapiro–Wilk test and were confirmed to be normally distributed.
The responsiveness of the two tools for health status (i.e. CAT and SGRQ) was determined by computation of the effect size (ES). Responsiveness can be defined as being the ability to measure the clinically important change obtained from an outcome measure. The ES was computed as recommended by Field28 using the following equation:
where r is the ES and t and df are the values of the t-statistic and degrees of freedom computed in the paired samples t-test. The greater the ES, the greater is the responsiveness of that tool. To interpret this, scores greater than 0.2, 0.5 and 0.8 were used to represent the modest, moderate and great sensitivity, respectively.29
Results
Sixty patients accepted to participate and consenting participants were enrolled into a 12-week PR programme. Of the 60 patients recruited, 49 patients completed the full programme (6 females and 43 males). The reasons provided for not completing the PR programme were perceiving no benefit in participation (n = 3), personal reasons (n = 2) and transportation difficulties (n = 6). The mean age of study participants was that of 66 years (SD: 7.76), with a mean weight of 75 kg (SD: 14.97) and height of 164 cm (SD: 7.54).
Following a 12-week PR programme, significant reductions in mean scores were identified for both the SGRQ and CAT, indicating an improvement in the health status of COPD participants following rehabilitation as is graphically shown in figure 1. These improvements were also registered 4 months after completion of the PR programme at the 28th week time frame as is shown in Figure 2.
Figure 1.
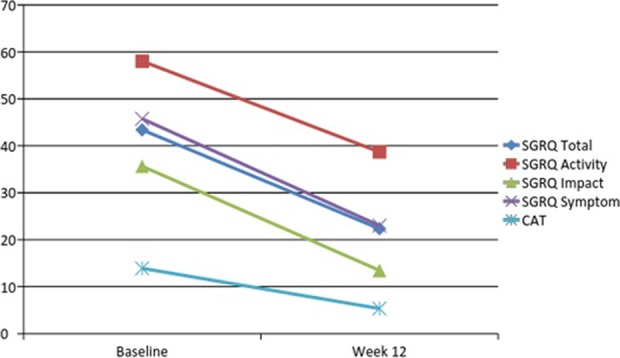
Graph showing changes in scores obtained with the SGRQ and the CAT at baseline and following a 12-week pulmonary rehabilitation programme. SGRQ: St. George’s Respiratory Questionnaire; CAT: chronic obstructive pulmonary disease assessment test.
Figure 2.
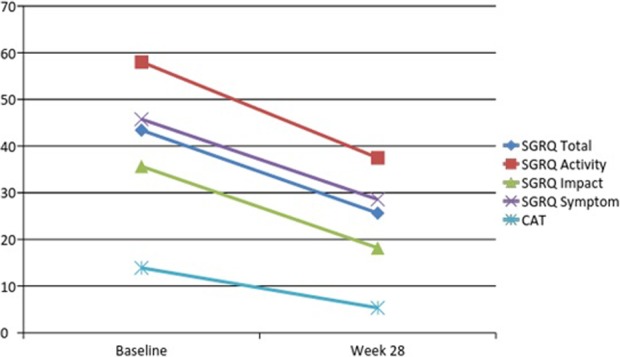
Change in scores for health status using the SGRQ and CAT between baseline and at follow-up (28 weeks). SGRQ: St. George’s Respiratory Questionnaire; CAT: chronic obstructive pulmonary disease assessment test.
As presented in Table 1, both the SGRQ (ES = 0.87) and CAT (ES = 0.75) displayed good responsiveness by total sample for the 12-week rehabilitation programme. Responsiveness for the three domains of SGRQ was the highest for the impact domain (ES = 0.81), followed by the symptom domain (ES = 0.76) and the activity domain (ES = 0.71), respectively, by total sample.
Table 1.
Change in health status measures between baseline and end of rehabilitation at 12 weeks.a
| Health status measure | Mean at baseline (SD) | Mean at end of PR (SD) | Mean change | SEM | ES | t (df) | |
|---|---|---|---|---|---|---|---|
| SGRQ total score | Total sample | 43.40 (14.88) | 22.33 (13.37) | 21.07 (11.81) | 1.69 | 0.87 | 12.49b (48) |
| mMRC 1–2 | 38.09 (13.18) | 18.69 (13.49) | 19.41 (12.25) | 2.27 | 0.85 | 8.53b (28) | |
| mMRC 3–4 | 51.10 (14.07) | 27.60 (11.57) | 23.50 (11.01) | 2.46 | 0.91 | 9.55b (19) | |
| SGRQ activity score | Total sample | 58.03 (18.97) | 38.68 (21.54) | 19.34 (19.35) | 2.76 | 0.71 | 6.99b (48) |
| mMRC 1–2 | 50.75 (16.28) | 32.01 (19.88) | 18.74 (20.58) | 3.82 | 0.68 | 4.90b (28) | |
| mMRC 3–4 | 68.57 (17.89) | 48.36 (20.57) | 20.21 (17.90) | 4.00 | 0.76 | 5.05b (19) | |
| SGRQ impact score | Total sample | 35.65 (17.81) | 13.45 (11.31) | 22.19 (15.98) | 2.28 | 0.81 | 9.721b (48) |
| mMRC 1–2 | 29.92 (14.63) | 10.93 (11.98) | 18.99 (13.13) | 2.44 | 0.83 | 7.79b (28) | |
| mMRC 3–4 | 43.96 (19.06) | 17.12 (9.37) | 26.85 (18.78) | 4.20 | 0.83 | 6.39b (19) | |
| SGRQ symptom score | Total sample | 45.76 (18.92) | 23.00 (16.65) | 22.76 (19.79) | 2.827 | 0.76 | 8.05b (48) |
| mMRC 1–2 | 41.93 (21.34) | 20.99 (17.22) | 20.94 (22.01) | 4.09 | 0.69 | 5.12b (28) | |
| mMRC 3–4 | 51.32 (13.36) | 25.93 (15.76) | 25.40 (16.23) | 3.63 | 0.85 | 6.99b (19) | |
| CAT total score | Total sample | 13.90 (8.903) | 5.33 (5.77) | 8.571 (7.539) | 1.077 | 0.75 | 7.96b (48) |
| mMRC 1–2 | 11.38 (6.15) | 4.14 (4.52) | 7.24 (5.84) | 1.084 | 0.78 | 6.68b (28) | |
| mMRC 3–4 | 17.55 (10.99) | 7.05 (6.98) | 10.50 (9.32) | 2.08 | 0.76 | 5.04b (19) | |
SD: standard deviation; PR: pulmonary rehabilitation; ES: effect size; df: degrees of freedom; SEM: standard mean error; t: t-statistic value; SGRQ: St. George’s Respiratory Questionnaire; mMRC: modified Medical Research Council; CAT: chronic obstructive pulmonary disease assessment test.
aSeverity of dyspnoea category: mild to moderate cases (mMRC 1–2) and severe to very severe cases (mMRC 3–4).
bSignificant at <0.001 level (one tailed).
An analysis of ESs demonstrates the greater responsiveness of the SGRQ (SGRQ: mMRC 1–2, ES = 0.85; mMRC 3–4, ES = 0.91) relative to the CAT (mMRC 1–2, ES = 0.78; mMRC 3–4, ES = 0.76) by dyspnoea severity category and for total score. The difference in ESs between the two tools (SGRQ: ES = 0.91; CAT: ES = 0.76) is most conspicuous for patients having higher dyspnoea severity scores (i.e. mMRC 3–4).
An analysis of the responsiveness of the SGRQ by domain and dyspnoea severity category indicates a similar responsiveness on the impact domain (i.e. mMRC 1–2, ES = 0.83; mMRC 3–4 ES = 0.83) and a higher responsiveness on the activity and symptom domains for patients having higher dyspnoea severity scores (mMRC 3–4: activity, ES = 0.76; symptom, ES = 0.85) relative to those in the mild to moderate dyspnoea category (mMRC 1–2: activity, ES = 0.68, symptom, ES = 0.69).
At the 28-week follow-up, the SGRQ (ES = 0.75) and the CAT (ES = 0.74) demonstrated similar responsiveness by total score (Table 2). Responsiveness for the three domains of the SGRQ was the highest for the impact domain (ES = 0.71), followed by the activity domain (ES = 0.68) and the symptom domain (ES = 0.64), respectively (Table 2).
Table 2.
Mean scores, significance of change (t-test) and ESs from baseline to the 28-week follow-up.a
| Health status measure | Mean at baseline (SD) | Mean at follow-up (28 weeks) | Mean change | SEM | ES | t | |
|---|---|---|---|---|---|---|---|
| SGRQ total score | Total sample | 43.40 (14.88) | 25.63 (15.42) | 17.77 (15.96) | 2.28 | 0.75 | 7.79b (48) |
| mMRC 1–2 | 38.09 (13.18) | 19.58 (12.26) | 18.51 (13.66) | 2.54 | 0.66 | 7.30b (28) | |
| mMRC 3–4 | 51.10 (14.07) | 28.30 (16.45) | 22.80 (13.96) | 3.12 | 0.74 | 7.30b (19) | |
| SGRQ activity score | Total sample | 58.03 (18.97) | 37.48 (19.63) | 20.54 (22.45) | 3.21 | 0.68 | 6.41b (48) |
| mMRC 1–2 | 50.75 (16.28) | 29.34 (18.41) | 21.41 (23.34) | 4.33 | 0.68 | 4.94b (28) | |
| mMRC 3–4 | 68.57 (17.89) | 43.68 (20.49) | 24.89 (19.45) | 4.35 | 0.79 | 5.72b (19) | |
| SGRQ impact score | Total sample | 35.65 (17.81) | 18.18 (14.35) | 17.47 (17.62) | 2.52 | 0.71 | 6.94b (48) |
| mMRC 1–2 | 29.92 (14.63) | 12.92 (10.04) | 17.00 (12.69) | 2.36 | 0.81 | 7.22b (28) | |
| mMRC 3–4 | 43.96 (19.06) | 18.82 (15.93) | 25.14 (17.63) | 3.94 | 0.83 | 6.38b (19) | |
| SGRQ symptom score | Total sample | 45.76 (18.92) | 28.56 (18.88) | 17.20 (20.75) | 2.96 | 0.64 | 5.80b (48) |
| mMRC 1–2 | 41.93 (21.34) | 23.90 (19.10) | 18.03 (20.93) | 3.89 | 0.66 | 4.64b (28) | |
| mMRC 3–4 | 51.32 (13.35) | 30.85 (18.55) | 20.47 (17.48) | 3.908 | 0.77 | 5.24b (19) | |
| CAT total score | Total sample | 13.52 (8.61) | 5.24 (5.79) | 8.29 (7.99) | 1.23 | 0.74 | 6.71b (41) |
| mMRC 1–2 | 11.38 (6.15) | 4.28 (4.54) | 7.10 (7.00) | 1.30 | 0.72 | 5.46b (28) | |
| mMRC 3–4 | 17.55 (10.99) | 8.30 (8.08) | 9.25 (9.51) | 2.13 | 0.71 | 4.35b (19) | |
SD: standard deviation; PR: pulmonary rehabilitation; ES: effect size; df: degrees of freedom; SEM: standard mean error; t: t-statistic value; SGRQ: St. George’s Respiratory Questionnaire; mMRC: modified Medical Research Council; CAT: chronic obstructive pulmonary disease assessment test.
aSeverity of dyspnoea category: mild to moderate cases (mMRC 1–2) and severe to very severe cases (mMRC 3–4).
bSignificant at <0.001 level (one tailed).
However, an analysis of the responsiveness of the tools by dyspnoea severity category for total score indicates that for the mild to moderate grouping (i.e. mMRC 1–2), the CAT (ES = 0.72) has a higher responsiveness than the SGRQ (ES = 0.66). However, for patients having higher dyspnoea scores (mMRC 3–4), the responsiveness of the SGRQ (ES = 0.74) was greater than the CAT (ES = 0.71) for total score. Furthermore, a greater responsiveness was identified in all domains of the SGRQ for patients having higher dyspnoea severity scores (mMRC 3–4: impact, ES = 0.83; activity, ES = 0.79; symptom, ES = 0.77) relative to those having milder dyspnoea scores (mMRC 1–2: impact, ES = 0.81; activity, ES = 0.68; symptom, ES = 0.6).
Discussion
This article contributes to the academic literature by examining the responsiveness of two measures (SGRQ and CAT) of health status, with a sample of COPD patients undergoing a 12-week PR programme and on follow-up at 28 weeks. The identification of a tool which is most responsive to changes is important, as health status is a predictor of COPD exacerbation, hospitalization, mortality and higher healthcare expenditure, due to higher medical needs and hospital doctor consultations.30
The results concur with those obtained in various studies (Chaplin et al.,31 Dodd et al.,32 Kon et al.33 and Jones et al.34), which demonstrates the good responsiveness of both tools in assessing the health status of COPD patients. However, this study contributes to the existing literature as it examines the responsiveness of the SGRQ and the CAT by severity of dyspnoea category. Such information is relevant when considering that higher levels of dyspnoea in COPD patients and the perceived benefit of such PR programmes predict both attendance and completion of PR programmes. Hence, the identification of a tool with a greater responsiveness to detect the change in health status by severity of the dyspnoea category could enable the introduction of individualized programmes in COPD patients.26
Highlighting that this article contributes to available literature by comparing the responsiveness of the CAT and SGRQ by severity of dyspnoea category, greater ESs were obtained with the SGRQ at the end of the 12-week PR programme. The difference in ESs is most apparent for patients with severe to very severe dyspnoea (mMRC 3–4: SGRQ = 0.91; CAT = 0.76). This may be explained as the factors contributing to the CAT scores differ slightly to those for the SGRQ.35 Thus, the greater responsiveness of the SGRQ at the end of PR may arise as physiologic measures (relating to greater severity of dyspnoea) such as airflow limitation and exercise capacity are significant contributors to variance for the total scores of the SGRQ but not for the CAT. Furthermore, the study has demonstrated that the specific domains of the SGRQ also show good responsiveness, providing the clinician with information regarding changes experienced in relation to symptoms, activity and impact of the disease. Additionally, at the end of the 12-week PR programme, the impact domain showed the highest responsiveness and the activity domain showed the least responsiveness by total sample and for the mMRC 1–2 category (mild–moderate dyspnoea). On the other hand, for participants in the severe to very severe dyspnoea category (mMRC 3–4), the symptom domain showed the highest responsiveness (ES = 0.85) and the activity domain showed the least responsiveness (ES = 0.76). This is interesting as severity of breathlessness is a predictor of non-adherence to PR programmes and so the use of the SGRQ enables the clinician to assess any symptoms which might gauge the progression of treatment for that patient. Moreover, the levels of various symptoms experienced by COPD patients can be predictors for COPD exacerbations and increased risk of mortality.30 Thus, the findings from this study demonstrate that although both tools have good responsiveness at the end of PR, the SGRQ provides an additional advantage of displaying a greater responsiveness, especially in relation to patients with severe to very severe dyspnoea (mMRC 3–4). This identification of a tool which can detect a change in health status is of importance, considering that severity of dyspnoea and perceived benefit of PR influence non-completion of PR programmes. Furthermore, the good responsiveness obtained for the specific domains on the SGRQ would also enable the provision of individual management care plans which not only target the individual’s health status in general but which may also target the individual domains.
The results obtained at the 28-week follow-up period demonstrate that both tools show similar levels of responsiveness (SGRQ, ES = 0.75; CAT, ES = 0.74). Moreover, the ‘impact’ domain had the highest responsiveness and the ‘symptom’ domain showed the least responsiveness by total sample and dyspnoea severity categories (i.e. mMRC group 1–2 and mMRC group 3–4). Therefore, both at the end of rehabilitation and at 28-week follow-up, the impact domain had the highest responsiveness, except for the mMRC 3–4 category where the symptom domain showed the highest responsiveness at the end of the 12-week rehabilitation programme. This information underscores the importance to the clinician of understanding the independent contributions of these domains, as well as the differences in responsiveness of these domains depending on their severity of dyspnoea category. Thus, both the SGRQ and CAT can be used to detect changes in health status at 28-week follow-up, although the SGRQ provides additional information in relation to the specific domains.
The need to select an appropriate tool to evaluate health status is relevant as it well documented25,30 that a poor health status is associated with adverse outcomes among which depression.36 In addition, depression has been demonstrated to be a significant predictor of non-completion of PR programmes, which are so essential in the care of persons with COPD.24
Thus, the present study highlights the greater responsiveness of the SGRQ relative to the CAT, in particular with patients having severe dyspnoea at the end of the PR programme, whereas a similar level of responsiveness, for both the CAT and SGRQ, was obtained at the 28-week follow-up period. Hence, by identifying changes in health status in such patients, one may provide individualized programmes which are responsive to their needs.
A limitation of this study is the small sample size. Although the research is conducted at the only general state hospital with a national capture potential, it is recommended by the authors that this study is repeated in order to analyse the trend identified with a larger sample size, hence enabling generalization. Furthermore, the sample in this study is somewhat biased in favour of male participants rather than females, owing to the opportunistic and ethical influences on the data collection process
Conclusion
This study demonstrates that both the CAT and SGRQ have good responsiveness and can detect changes in health status for COPD patients. However, an analysis of results by severity of dyspnoea indicates the greater responsiveness of the SGRQ, especially in relation to patients presenting as severe cases of dyspnoea (mMRC 3–4) for COPD at the end of PR programme, whereas at 28-week follow-up, both tools show similar responsiveness by dyspnoea category and by total sample.
Acknowledgements
The authors would like to thank all the participants who participated in this study and the CEO of the state general hospital for his support.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Chan-Yeung M, Ait-Khaled N, White N, et al. The burden and impact of COPD in Asia and Africa [state of the art]. Int J Tuberc Lung Dis 2004; 8(1): 2–14. [PubMed] [Google Scholar]
- 2. Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004; 364(9434): 613–620. [DOI] [PubMed] [Google Scholar]
- 3. Bolton CE, Bevan-Smith EF, Blakey JD, et al. British thoracic society guideline on pulmonary rehabilitation in adults. Thorax 2013. September; 68 Suppl 2: ii1–30. [DOI] [PubMed] [Google Scholar]
- 4. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370(9589): 765–773. [DOI] [PubMed] [Google Scholar]
- 5. Tsukino M, Nishimura K, Ikeda A, et al. Physiologic factors that determine the health-related quality of life in patients with COPD. Chest 1996; 110(4): 896–903. [DOI] [PubMed] [Google Scholar]
- 6. Bendtsen P, Leijon M, Sofie Sommer A, et al. Measuring health-related quality of life in patients with chronic obstructive pulmonary disease in a routine hospital setting: feasibility and perceived value. Health Qual Life Outcomes 2003; 11(1): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4): 347–365. [DOI] [PubMed] [Google Scholar]
- 8. Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992; 145(6): 1321–1327. [DOI] [PubMed] [Google Scholar]
- 9. St. George’s Respiratory Questionnaire original English version, http://www.healthstatus.sgul.ac.uk/SGRQ_download/Original%20English%20version.pdf (accessed 5 February 2015).
- 10. Kaltsakas G, Travlos A, Rovina N. Improving the process of care in chronic obstructive pulmonary disease: the COPD assessment test (CAT) in the armature of the tools assessing COPD. Pneumon 2011; 24(3): 286. [Google Scholar]
- 11. COPD Assessment Test, 2009, www.catestonline.org/images/pdfs/CATest.pd f (accessed 5 February 2015).
- 12. Peruzza S, Sergi G, Vianello A, et al. Chronic obstructive pulmonary disease (COPD) in elderly subjects: impact on functional status and quality of life. Respir Med 2003; 97(6): 612–617. [DOI] [PubMed] [Google Scholar]
- 13. Doll H, Duprat-Lomon I, Ammerman E, et al. Validity of the St George’s respiratory questionnaire at acute exacerbation of chronic bronchitis: comparison with the Nottingham health profile. Qual life Res 2003; 12(2): 117–132. [DOI] [PubMed] [Google Scholar]
- 14. Singh S, Sodergren S, Hyland M, et al. A comparison of three disease-specific and two generic health-status measures to evaluate the outcome of pulmonary rehabilitation in COPD. Respir Med 2001; 95(1): 71–77. [DOI] [PubMed] [Google Scholar]
- 15. Glaab T, Vogelmeier C, Buhl R. Outcome measures in chronic obstructive pulmonary disease (COPD): strengths and limitations. Respir Res 2010; 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J 2011; 38(1): 29–35. [DOI] [PubMed] [Google Scholar]
- 17. Tsiligianni IG, van der Molen T, Moraitaki D, et al. Assessing health status in COPD. A head-to-head comparison between the COPD assessment test (CAT) and the clinical COPD questionnaire (CCQ). BMC Pulm Med 2012; 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wedzicha JA, Bestall JC, Garrod R, et al. Randomized controlled trial of pulmonary rehabilitation in severe chronic obstructive pulmonary disease patients, stratified with the MRC dyspnoea scale. Eur Respir J 1998; 12(2): 363–369. [DOI] [PubMed] [Google Scholar]
- 19. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000; 355(9201): 362–368. [DOI] [PubMed] [Google Scholar]
- 20. Guyatt GH, Berman LB, Townsend M, et al. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987; 42(10): 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beaton DE, Bombardier C, Katz J, et al. A taxonomy for responsiveness. J Clin Epidemiol 2001; 54(12): 1204–1217. [DOI] [PubMed] [Google Scholar]
- 22. Jones G, Radley S, Lumb J, et al. Responsiveness of the electronic personal assessment questionnaire-pelvic floor (ePAQ-PF). Int Uro gynec J 2009; 20(5): 557–564. [DOI] [PubMed] [Google Scholar]
- 23. van der Lee, Johanna H, Beckerman H, et al. The responsiveness of the action research arm test and the Fugl-Meyer assessment scale in chronic stroke patients. J Rehabil Med 2001; 33(3): 110–113. [DOI] [PubMed] [Google Scholar]
- 24. Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis 2011; 8(2): 89–99. [DOI] [PubMed] [Google Scholar]
- 25. Sabit R, Griffiths TL, Watkins AJ, et al. Predictors of poor attendance at an outpatient pulmonary rehabilitation programme. Respir Med 2008; 102(6): 819–824. [DOI] [PubMed] [Google Scholar]
- 26. Holzhauer-Barrie J, Lowe D, Searle L, et al. Clinical outcomes of pulmonary rehabilitation. Results for the UK National COPD Audit In: B109. Highlights and advances in pulmonary rehabilitation 2016 May. Royal College of Physicians, London: American Thoracic Society, pp. A4515–A4515. [Google Scholar]
- 27. Nici L, Donner C, Wouters E, et al. American thoracic society/European respiratory society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173(12): 1390–1413. [DOI] [PubMed] [Google Scholar]
- 28. Field A. Discovering statistics using SPSS. Sage publications limited, 2009. [Google Scholar]
- 29. Smith HJ, Taylor R, Mitchell A. A comparison of four quality of life instruments in cardiac patients: SF-36, QLI, QLMI, and SEIQoL. Heart 2000; 84(4): 390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu M, Zhao Q, Chen Y, et al. Quality of life and its association with direct medical costs for COPD in urban China. Health Qual Life Outcomes 2015; 13(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chaplin E, Gibb M, Sewell L, et al. Response of the COPD assessment tool in stable and postexacerbation pulmonary rehabilitation populations. J Cardiopulm Rehabil Prev 2015; 35(3); 214–218. [DOI] [PubMed] [Google Scholar]
- 32. Dodd JW, Hogg L, Nolan J, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax 2011; 66(5): 425–429. [DOI] [PubMed] [Google Scholar]
- 33. Kon SS, Dilaver D, Mittal M, et al. The Clinical COPD Questionnaire: response to pulmonary rehabilitation and minimal clinically important difference. Thorax 2014; 69(9): 793–798. [DOI] [PubMed] [Google Scholar]
- 34. Jones PW, Harding G, Wiklund I, et al. Tests of the responsiveness of the COPD assessment test following acute exacerbation and pulmonary rehabilitation. Chest 2012; 142(1): 134–140. [DOI] [PubMed] [Google Scholar]
- 35. Morishita-Katsu M, Nishimura K, Taniguchi H, et al. The COPD assessment test and St. George’s Respiratory Questionnaire: are they equivalent in subjects with COPD? Int J Chron Obstruct Pulmon Dis 2016; 11: 1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schlecht NF, Schwartzman K, Bourbeau J. Dyspnea as clinical indicator in patients with chronic obstructive pulmonary disease. Chron Respir Dis 2005; 2(4): 183–191. [DOI] [PubMed] [Google Scholar]


