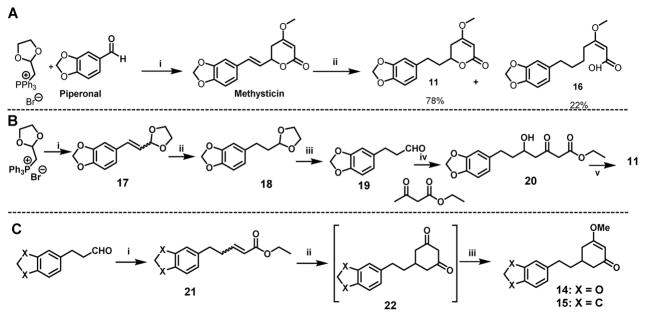
Scheme 3.
The synthesis of 11 – 15. (A) The first synthetic route for 11 with 16 being a by-product: (i) (a) (1,3-dioxolan-2-yl)methyl triphenylphosphonium bromide, LiOMe, 70 °C 6h; (b) 1N HCl, THF, RT, 2h; (c) Ethyl acetoacetate, NaH, n-BuLi, THF −78°C to RT; (d) K2CO3/methanol, then dimethylsulfate/acetone; (ii) H2, Pd/C, THF. (B) The second synthetic route of 11: (i) LiOMe, dry THF, 0 °C, piperonal, 70 °C, 6 h; (ii) H2, Pd/C (1 atm), THF, 2 h; (iii) 1N HCl, THF, RT, 2 h; (iv) (a) NaH, n-BuLi, dry THF, 0 °C, 30 min, (b) −55 °C to RT, 6h; (v) (a) K2CO3, MeOH, RT, 6h, (b) Dimethyl sulfate, acetone, RT, 12 h. (C) The synthetic route of 14 and 15: (i) (carbethoxymethylene)triphenylphosphorate, THF, reflux, 12h; (ii) NaH, acetone, THF and toluene, 0 °C-RT, 4h; (iii) Dimethyl sulfate, acetone, RT, 12 h.