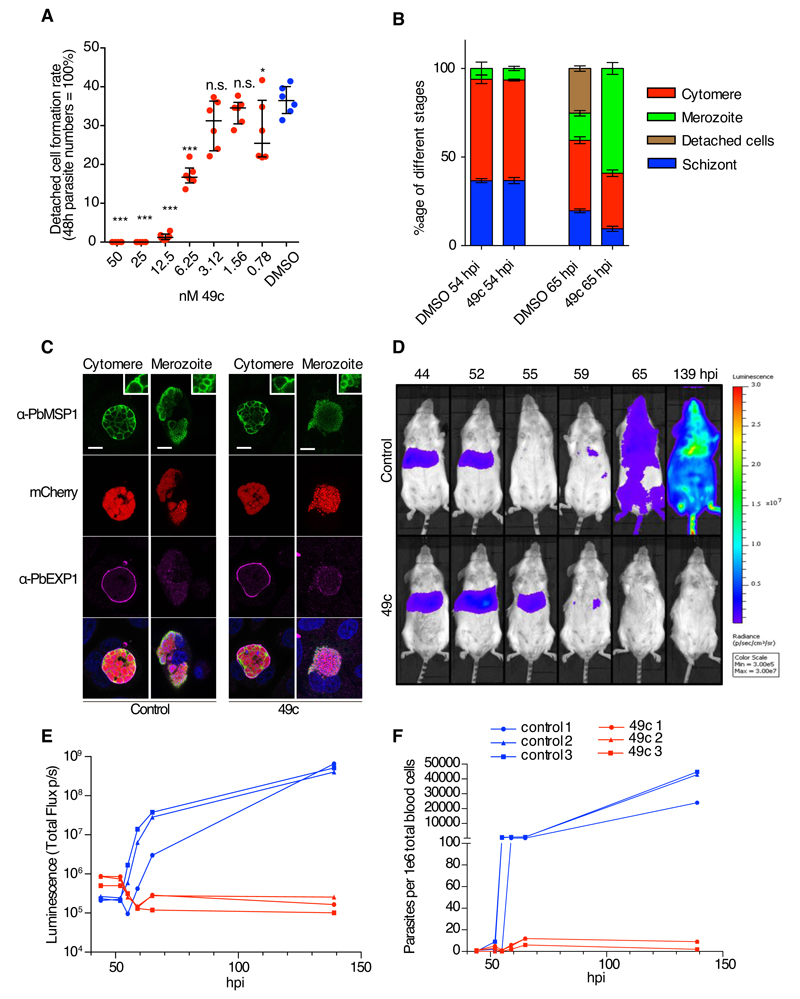
Fig 4. Compound 49c prevents hepatic merosomes formation.
A) The detached cell formation rate was reduced in 49c-treated P. berghei-infected HeLa cells in a dose-dependent manner. The results were statistically evaluated by a one-way ANOVA test with Dunnet’s Multiple Comparisons (* p ≤ 0.05, ** p ≤ 0.01 *** p ≤ 0.001, ns/not significant > 0.05). B) Distribution of the different hepatic stages by IFA of mCherry-expressing P. berghei in HeLa cells treated with 49c/DMSO 54 h and 65 h post infection. An anti-Msp1 antiserum was used to detect maturation of merozoites. Classification was done as reported before (41). C) Positive MSP1 staining of infected HeLa cells 65 h post infection indicates normal merozoite development. At the cytomere stage, clear invaginations are visible and at the merozoite stage, individual merozoites are labelled with an MSP1 antibody. Exp1 is shown to visualize the PVM, (bar: 10μm, inserts are magnified 3x). D) Mice were infected by i.v. injection mCherry/Fluc expressing P. berghei sporozoites, and treated with 100 mg/kg 49c at 20 and 40 hpi (n=3). At indicated time points Rediject-D-Luciferin was administered and whole body luminescence detected using the in vivo imaging system (IVIS). In control mice, the luminescence signal from the liver disappears at 55 hours, the time when hepatic parasites egress. From 65 hpi blood stage parasites are detectable. In drug-treated mice the signal increased at 52 hpi and then remained longer in the liver (still present at 55 hpi) most likely because egress is blocked. E) The measured total flux (photons/second) in the head/chest region (blood stage parasites) of the mice in D is shown for the different time points. F) At each time point blood samples were analysed by FACS, detecting the mCherry signal of the parasite in 106 whole blood cells confirming the results obtained by bioluminescence imaging (E).