Fig. 3.
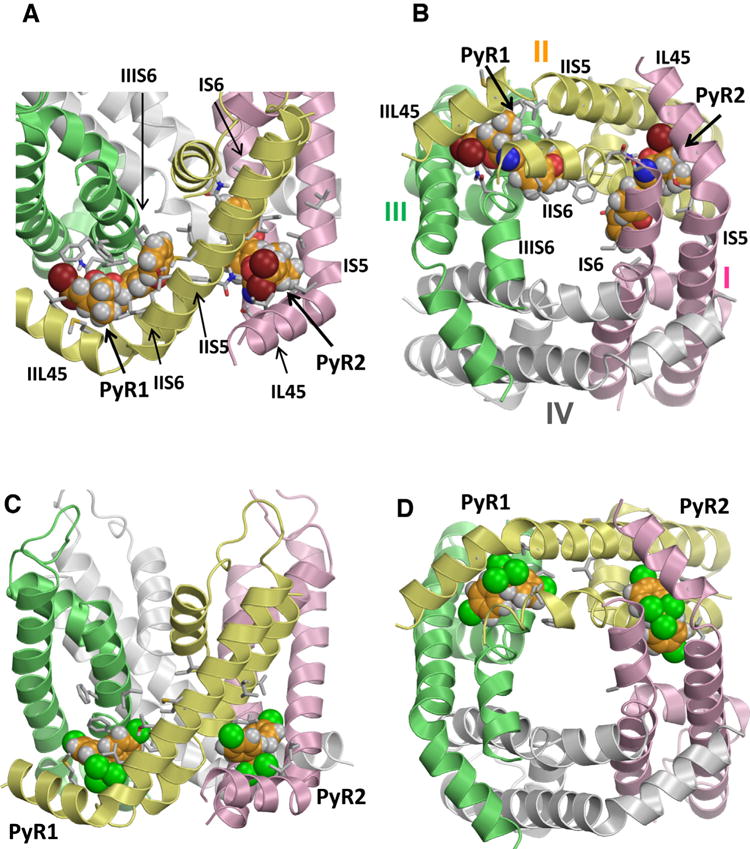
Kv1.2-based model of the open AaNav1-1 channel pore module with two DMT molecules (A,B) and two DDT molecules (C,D) docked into PyR2 and revised PyR1 sites. Helices in domains I, II, III and IV are shown by pink, yellow, green and white ribbons, respectively. Arrows point to the kink regions between helices L45 and S5 of respective receptors. Known pyrethroid-sensing residues are shown as sticks and respective helices are labeled. Carbon, oxygen, nitrogen and hydrogen atoms of the ligands are, respectively, orange, red, blue, and gray. Bromine atoms in DMT are brown and chlorine atoms in DDT are green. Note that the bulkiest moieties of the insecticides (dimethylcyclopyl group of DMT and trichloromethyl group of DDT) bind similarly between L45, S5 and S6 helices, while aromatic substituents of the bulkiest groups extend between two domains. Figures A and B are Reproduced with permission from Molecular Pharmacology (Du Y, Nomura, Y, Zhorov BS, and Dong K. Rotational symmetry of two pyrethroid receptor sites in the mosquito sodium channel. 2015; 88: 273–280). Figures C and D are originally published in the Journal of Biological Chemistry (Du et al., 2016). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
