Abstract
Most primates live in habitats with some level of anthropogenic disturbance, and such disturbances have a larger impact on frugivorous primates that are more sensitive to ecological disruptions than folivores. Fecal glucocorticoid metabolites provide insight into how the external environment affects internal physiological state, and thus provide information on how anthropogenic pressures become embodied. Here, I examine how subgroup size and glucocorticoids vary with high and low fruit abundance, and how fruit abundance, subgroup size, and activity budget affect fecal glucocorticoid metabolites in female spider monkeys (Ateles geoffroyi) living in an anthropogenically disturbed habitat. I measured these variables via behavioral, ecological, and fecal sampling for 15 months in 17 female spider monkeys at El Zota Biological Field Station. Subgroup size was significantly larger during periods of high fruit abundance, but glucocorticoids did not differ between periods of low and high fruit abundance. Monthly fruit abundance predicted subgroup sizes significantly, but did not predict fecal glucocorticoid concentrations. Increased resting time and reproductive state predicted fecal glucocorticoid concentrations significantly, but travel and foraging time had no significant effect on glucocorticoid concentrations. Individual resting time over the study period correlated negatively with glucocorticoid concentrations. These results suggest that spider monkeys cope with variation in fruit abundance by adjusting subgroup size, and that these adjustments may mitigate environmental stress in this mildly seasonal environment. The large, relatively productive forest size at this site, and the availability of anthropogenic food sources, enable this population of spider monkeys to cope with human-induced habitat disturbance.
Keywords: Anthropogenic habitats, Ateles, Fission–fusion, Glucocorticoids, Subgrouping
Introduction
Anthropogenic disturbances threaten primates in a variety of habitats, and are the major threat driving primate populations to extinction (Estrada et al. 2017; McKinney 2015). Anthropogenic disturbance alters or intensifies the effects of ecological challenges and social dynamics on primate physiological stress and health (González-Zamora et al. 2011; Rimbach et al. 2014). Thus, describing the impact of anthropogenic disturbances across habitats is crucial to understanding how threatened primate species cope with these disturbances (Estrada et al. 2017; McKinney 2015; Rangel-Negrín et al. 2009; Rimbach et al. 2014). Tropical frugivores are at particular risk of population decline because of their dependence on large, intact tracts of forest and sensitivity to habitat degradation (González-Zamora et al. 2011; McKinney 2015; Sorensen and Fedigan 2000) and females are more sensitive to ecological stressors than males because of the energetic demands of pregnancy and lactation (Foerster et al. 2012; Gaulin and Sailer 1985; Trivers 1972; Wrangham 1980).
Measurement of fecal glucocorticoid metabolites provides insight into how anthropogenic pressures such as habitat disturbance affect physiology (Martínez-Mota et al. 2007; McEwen and Wingfield 2003). Glucocorticoids are metabolic hormones that mediate allostatic balance (McEwen and Wingfield 2003; Nelson and Drazen 2007; Sapolsky 1992). They promote energy usage by increasing availability of glucose in the bloodstream, and are crucial in regulating feeding behavior, locomotion, and energy metabolism (Landys et al. 2006). When glucocorticoids rise to prepare for acute challenges, or predictable seasonal stressors, this response is adaptive (McEwen and Wingfield 2003; Nelson and Drazen 2007 Sapolsky 1992). However, when animals are under continual stress, dysregulation of the hypothalamic–pituitary–adrenal axis may result in chronically elevated glucocorticoid concentrations or suppressed glucocorticoid activity, which can compromise health, particularly immune and reproductive function (McEwen and Wingfield 2003; Sapolsky 1992). When anthropogenic pressures alter food availability, it can make it harder for primates to obtain preferred food items, thus increasing environmental stress (González-Zamora et al. 2011; Martínez-Mota et al. 2007; McKinney 2015; Sorensen and Fedigan 2000). Such pressures can result in altered diets or activity budgets, elevated glucocorticoids, and compensatory behaviors such as foraging on crops (González-Zamora et al. 2011; Hockings et al. 2012; Martínez-Mota et al. 2007; McKinney, 2011; Rangel-Negrín et al. 2009). For example, Geoffroy’s spider monkeys (Ateles geoffroyi) and black howler monkeys (Ateles pigra) living in fragmented forests exhibit higher glucocorticoids than those living in continuous, conserved forest (Martínez-Mota et al. 2007; Rangel-Negrín et al. 2009).
In species with high levels of fission–fusion dynamics, subgroup size typically varies with fruit availability (chimpanzees [Pan troglodytes]: Basabose 2004; Chapman et al. 1995; Itoh and Nishida 2007; Matsumoto-Oda et al. 1998; bonobos [Pan paniscus]: Mulavwa et al. 2008; spider monkeys [Ateles spp.]: Asensio et al. 2008, 2009; Chapman 1990; Chapman et al. 1995; Schaffner et al. 2012; Shimooka 2003; Weghorst 2007; black-and-white ruffed lemur [Varecia variegata]: Baden et al. 2016; red-capped mangabeys [Cercocebus torquatus]: Dolado et al. 2016). However, some chimpanzee and bonobo groups do not exhibit this relationship (Moscovice et al. 2007; Wakefield 2008), or exhibit mixed results (Hashimoto et al. 2003; White 1998), and the presence of females with anogenital swellings also affects subgroup size (Matsumoto-Odo et al. 1998). Female spider monkeys, particularly those with dependent offspring, typically range in smaller subgroups than males (Chapman 1990; Symington 1990). Smaller subgroups reduce feeding competition and allow individuals to spend more time foraging at each resource. As subgroup size increases, animals must travel further to find adequate food resources for the entire subgroup (Asensio et al. 2009; Lehmann et al. 2007; Suarez 2006). Thus, subgroups may be larger when resources are more abundant, or when larger resources patches are available (Asensio et al. 2009; Chapman et al. 1995; Weghorst 2007). However, in a study of brown spider monkeys (Ateles hybridus) living in a fragmented forest, spider monkeys were observed in larger parties when resources were scarce (Rimbach et al. 2014). Larger subgroups may provide more opportunities for affiliation, but also increase risks of competition and aggression.
The highly frugivorous Geoffroy’s spider monkey is Endangered, and understanding how anthropogenic disturbances affect the species is crucial to conservation efforts (Cuarón et al. 2008; Di Fiore and Campbell 2007; Rangel-Negrín et al. 2009). Understanding these effects is complicated by their high levels of fission–fusion dynamics (Aureli et al. 2008; Chapman 1990; Chapman et al. 1995; Symington 1990). Although monkeys adjust grouping patterns to cope with reduced food availability, such adjustments intensify social pressures (Asensio et al. 2008, 2009; Chapman 1990; Hartwell et al. 2014; Rimbach et al. 2014; Symington 1990). Thus, anthropogenic disturbance potentially affects stress through two mechanisms. First, it could affect food availability, leading to nutritional stress, or it could affect physiological stress, by altering activity budgets and social dynamics.
Here, I examine patterns of fecal glucocorticoid metabolite concentrations in wild female Geoffroy’s spider monkeys living in an anthropogenically modified rainforest habitat. I collected data on fruits consumed, fruit abundance, subgroup size, activity variables, and fecal glucocorticoid metabolites from females in one habituated community for 15 mo. Because anthropogenic disturbance can indirectly affect stress by altering subgrouping patterns, I hypothesized that subgroup sizes and glucocorticoid concentrations would vary in response to fruit abundance, and that fruit abundance would be a stronger predictor of glucocorticoid concentrations than subgroup size or activity variables. I predicted that glucocorticoid concentrations would be higher during periods of low fruit abundance compared to periods of high fruit abundance, and that subgroup sizes would be largest during periods of high fruit abundance. Additionally, because anthropogenic disturbance can indirectly affect stress via altered activity budgets, I predicted that increased time engaged in foraging and traveling would be associated with higher glucocorticoid concentrations, while increased resting time would be associated with lower glucocorticoid concentrations.
Methods
Study Site
I collected data at El Zota Biological Field Station in Costa Rica, from June 2010 to August 2011. El Zota is a 1000-ha private reserve situated in the northeastern region of the country at 10°57.6 N, 83°75.9′W ca. 20 km from Tortuguero National Park and Barro del Colorado Reserve (Lindshield 2006; Pruetz and LaDuke 2001). This tropical wet forest (Gentry 1982; González-Zamora et al. 2011) receives ca. 4000–5000 mm of rainfall annually and exhibits very mild seasonality (Sanford et al. 1994; Wolfe and Ralph 2009). This region does not experience true dry seasons, as defined as <100 mm rainfall (Suarez 2006).
Two Geoffroy’s spider monkey communities are present at El Zota: one community ranges in the northern primary forest, and the other ranges in the southern secondary forest (Lindshield 2006; Pruetz and LaDuke 2001; Rodrigues 2007, 2014; Rodrigues et al. 2015). I studied the well-habituated Pilón community, which ranges in the southern secondary forest adjacent to the field station. The secondary forest contains thick undergrowth consisting of palms and herbaceous vegetation, including walking palm (Socretea) and false bird of paradise (Heliconia). The forest has abundant gallery forest due to the presence of many streams and creeks, and consists of fig (Ficus) and guaba/ice cream bean (Inga), similar to other riparian habitats (Lindshield 2006). The secondary forest has a mosaic pattern of disturbance, with harvestable monocultures of exotic beechwood (Gmelina aborea) and native balsa (Ochroma pyramidale) along the main road, with scattered crabwood (Carapa guianensis), pílon (Hyeronima alchorneoides), and spanish elm (Cordia alliodora: Lindshield 2006; Pruetz and LaDuke 2001). The southern portion of the station also includes banana and plantain (Musa) plantation which is left for wildlife to forage in, a vegetable and fruit garden that is used by monkeys foraging for bananas and guava (Psidium), and former pastureland (Lindshield 2006; pers. obs.). The anthropogenic disturbance is classified as DCH1B (McKinney 2015), corresponding to a landscape that is protected but has some roads and minor resource extraction (D), a diet predominantly based on wild foods with opportunistic crop-foraging (C), researcher and tourist presence that fluctuates over the year (H1), and reduced natural predators with no human predation (B: Lindshield 2006; McKinney 2015).
Behavioral Data and Fecal Sample Collection
I collected behavioral data on all adult and subadult females present in the Pilón community. These 17 females were part of a community containing 39–41 individuals, including the females, 15 immature individuals, and 7–9 adult and subadult males (Rodrigues et al. 2015). I determined reproductive condition retrospectively based on births and nursing. My focal subjects were seven females that were cycling, six that were lactating, two that transitioned from late lactation to cycling, and two that were pregnant (Rodrigues et al. 2015). I observed one female (JI) during pregnancy and early lactation, and one female (ST) during cycling, pregnancy, and early lactation. I conducted 10-min scan samples (Altmann 1974) of party size and composition and 10-min focal periods with 2-min instantaneous sampling intervals on the females between 05:30 h and 18:00 h, with all-occurrence recording of social behavior during focal samples. When I encountered a subgroup, I identified the females of the party and selected a focal subject. Initially, I chose the first female I identified to follow first; however, after data collection became unequal, I chose the least-sampled for the first focal follow. Subsequently, I followed every other female in the party, sampling each female in the subgroup. If only one female focal subject was present, I conducted consecutive follows to maximize fecal data collection. I excluded focal periods from analysis if individuals were out of sight for more than three instantaneous recordings during the focal period. During focal periods, I collected the following information: 1) time of day, 2) location in the forest (trail or nearest trails), 3) identity and activity of focal animal, 4) type of social interaction, and 5) initiator/recipient of social interaction. The activity categories I included were feeding/foraging, resting, traveling, social behavior, and other behaviors. I divided all-occurrence observations of social behaviors into affiliative behaviors (grooming, social play, embrace, huddle, touch) and aggressive behaviors (chase, harass, fight). I collected behavioral data on write-in-the-rain check sheets and entered it daily into Excel® files. I used activity data from instantaneous focal animal sampling periods to generate individual overall activity budgets as well as individual monthly activity budgets. I spent an estimated time of 715 contact hours with spider monkey subgroups and obtained 186 focal hours from 17 focal animals. I estimated contact time based on time in the field spent following monkey subgroups and collecting fecal samples. During focal and subgroup follows, I collected fecal samples using methods described previously (Rodrigues et al. 2015). Either I or a field assistant collected fecal samples using a plastic bag or plastic spatula and test tube. We immediately placed samples in a thermos with a cold pack, and returned to the field station within 1 h to process them.
Only 11 individuals were sampled for >5 h, so I limited monthly analyses to these individuals (Table I). Mean (± SD) observation hours for the 11 well-sampled individuals were 14.72 ± 5.40 per individual, comparable to those in other studies of spider monkeys (Ahumada 1992; Campbell 2000; Slater et al. 2009). I collected a mean of 11.27 ± 4.63 fecal samples per individual.
Table I.
Distribution of focal samples, hours of observation, and fecal samples per individuals for female Geoffroy’s spider monkeys (Ateles geoffroyi) at El Zota Biological Field Station, Costa Rica June 2011–August 2012
| ID | Focal periods | Observation hours | Fecal samples |
|---|---|---|---|
| AG | 62 | 10.3 | 5 |
| AS | 81 | 13.5 | 17 |
| AR | 115 | 19.2 | 13 |
| BU | 74 | 12.3 | 9 |
| HO | 79 | 13.2 | 9 |
| IS | 31 | 5.2 | 5 |
| JI | 75 | 12.5 | 8 |
| JL | 132 | 22.0 | 17 |
| LE | 140 | 23.0 | 18 |
| MC | 72 | 12.0 | 11 |
| RU | 110 | 18.5 | 12 |
When I encountered a spider monkey subgroup, I recorded subgroup composition and identified fruits consumed during the encounter to derive a list of fruits eaten during the study. When subgroup composition changed, I treated it as a new subgroup. I recorded all individually locomoting individuals as subgroup members. During 47% of subgroup follows (267 of 573), individuals ate identifiable fruits, and I used these observations to generate a list of the most frequently consumed fruits. These include Spondias, Hyeronima, Ficus, Musa, Socretea and Iriartea, Virola, Pithecoctenium, Inga, Brosimum, Psidium, Dipteryx, and Cercropia. In some cases, (<1% of subgroup encounters), I could not identify the fruits the monkeys consumed, and I excluded these fruits from analyses.
Ecological Data Collection
Primatologists commonly use trees’ diameter at breast height (DBH), ca. 1.4 m, to estimate fruit abundance and compare relative annual variation (Chapman et al. 1992; Pruetz 2009). DBH is a consistent predictor of fruit abundance that correlates with fruit biomass and is the most appropriate measure to use with multiple observers (Chapman et al. 1992). Estimates based on DBH are particularly useful in forest with high canopies that make visual estimation challenging (Pruetz 2009). Thus, I chose presence/absence of fruit on phenology transects in conjunction with DBH (Chapman et al. 1994) to minimize time away from behavioral and fecal data collection, ensure reliability among field assistants, and allow for comparison to other studies. I established twenty 2 m by 50 m plots throughout the secondary forest based on randomized global positioning coordinates (GPS), using a modified version of the 0.1-ha method (Gentry 1982). In some cases, I moved plot locations from the randomized coordinates to adequately sample all types of forest present (swamp, gallery, plantation), and to ensure we could traverse transects in all seasons (seasonal inundation makes some areas impassable). I marked and numbered all trees and lianas with DBH >10 cm in each plot. In August 2010, I measured and recorded the DBH of each tree in the plots. Whenever possible, I identified the genus of the tree, although many were not identifiable until fruiting. I used a comprehensive list of spider monkey feeding trees at El Zota compiled by Lindshield (2006) to identify feeding trees. Twice a month, my assistants and I assessed all marked trees in the transect plots for the presence/absence of fruit and flowers (Chapman et al. 1994). I calculated biweekly fruit abundance indexes by summing the DBHs (ΣDBH) of all trees with fruit. Although we recorded the presence of all fruit and flowers, I counted fruit only in the fruit abundance calculations, and I excluded fruits known to be inedible to the spider monkeys (Pentaclethera and Ochroma). Whenever possible, I identified the fruiting trees on these transects and used them to generate the list of available fruits (Table II).
Table II.
Fruit availability and consumption by female Geoffroy’s spider monkeys (Ateles geoffroyi) from September 2010–August 2011 in the secondary forest of El Zota Biological Field Station, Costa Rica.
| Fruits consumed | Fruit availability | % consumption |
|---|---|---|
| Hyeronima (pílon) | Sept, June–Aug | 38.2 |
| Spondias (hog plum) | Sept–Nov, Apr–Aug | 28.8 |
| Ficus (fig) | Sporadic (Nov/Jan/May) | 13.1 |
| Musa (banana) | Year-round | 4.1 |
| Socretea/Iriartea (walking palm) | Year-round | 4.1 |
| Virola (frutidorada) | May–June | 2.6 |
| Pithecoctenium (monkey comb) | Feb–Apr | 1.9 |
| Inga (ice cream bean) | Unknown | 1.9 |
| Brosimun (ojoche) | Apr | 1.9 |
| Psidium (guava) | Unknown | 1.5 |
| Dipteryx (almendra) | Dec–Mar | 1.1 |
| Cercropia (yarumo) | Year-round | 0.8 |
Hormonal Data Collection and Analysis
With field assistants, I processed and stored fecal samples using solid-phase extraction following Ziegler and Wittwer (2005), with modifications by Erin Ehmke (pers. comm.). We mixed fecal material (0.1 g) with 2.5 mL of distilled water and 2.5 mL of ethanol. We shook the mixture by hand for 5 min, hand-spun it for 10 min, and let it settle for ≥30 min. We removed and transferred ca. 3 mL of the supernatant to a clean test tube. Then, we removed 2 mL of the filtered supernatant and passed it through a Prevail C18 Maxi-Clean Solid Phase Extraction Cartridge (©Alltech, Lexington, KY). To prevent continued extraction, we washed cartridges with 2 mL of distilled water. To ensure preservation in the field, we stored cartridges in Ziploc bags with silica gel, and then placed them in a cooler with additional silica gel. I stored the cartridges for 72–460 days.
I sent samples for analysis at the Wisconsin National Primate Center Core Assay facility, using an in-house cortisol enzyme immunoassay. This assay cross-reacts with multiple glucocorticoids and we validated it for use with captive female Geoffroyi’s spider monkeys (Rodrigues et al. 2015). We washed cartridges with 1 mL of a 95:5 water: methanol solution. Next, we added 2 mL of methanol to the cartridge and collected. Then we dried the methanol, resuspended it in ethanol, and stored it in the refrigerator until we performed the assays. First, we evaporated 200 μL of the sample, resuspended it in 300 μL of horseradish peroxidase diluent at a concentration of 1:150,000, and plated it. We incubated the plates for 2 h, washed the unbound material off the plate, and added the substrate. We added stop solution after sufficient color developed. We fitted assays to a standard curve using log-logit regression. We validated the cortisol assay for accuracy (−93.49 ± 1.09, N = 6) and parallelism (−t = 1.14, df = 22, P = 0.13, N = 7) using internal controls to determine precision. The inter- and intraassay coefficients of variation for the two pools were 15.8/9 and 13.1/6. We previously established that estradiol had a significant effect on fecal glucocorticoid metabolite concentrations, but time of day and days in storage had no effect (Rodrigues et al. 2015).
Statistical Analysis
Because my sampling procedures compromised independence of results, I compared activity budgets from the full dataset to a dataset with results from consecutive focal periods omitted. Consecutive follows did not yield significantly different activity budgets than nonconsecutive follows (feed: t = 0.801, P = 0.44, rest t = −0.61, P = .952, social t = −0.770, P = .455, t = −1.06, P = −0.0917, df = 13). Because there was no difference between these samples, I used results from the full dataset for subsequent analyses.
I used Mann–Whitney U tests to examine differences in glucocorticoid concentration and subgroup size between periods with low fruit abundance (September–December) and high fruit abundance (January–August). I used data from all 17 focal individuals for this analysis. I set α at 0.05.
I used general linear mixed models (GLMMs) to examine the effects of fruit abundance and reproductive state on mean monthly subgroup size, and fruit abundance, activity budget variables, and reproductive state on mean monthly fecal glucocorticoid metabolite concentrations for the 11 individuals that were sufficiently sampled. Studies examining fecal glucocorticoids using GLMM frequently use monthly means (Foerster et al. 2012; Gesquiere et al. 2008; Girard-Buttoz et al. 2009; Weingrill et al. 2004). I used this procedure to avoid biasing data toward time periods with more frequent behavioral or fecal sampling. Because data were not normally distributed, I used a gamma probability distribution and a log link function. Reproductive state significantly affects measurement of glucocorticoid concentrations (Rodrigues et al. 2015). In the model for subgroup size, I incorporated reproductive state (categorical: cycling, lactating, or pregnant), and fruit abundance (continuous), as fixed factors, and ID as a random factor. In the model for fecal glucocorticoid metabolite concentration, I incorporated monthly mean percentages of rest, travel, feed, social (continuous), fruit abundance (continuous), subgroup size (continuous), and reproductive state (categorical) as fixed factors and ID as a random factor. To test for collinearity, I examined correlation matrices to ensure r < 0.5, and examined the variance inflation factor (VIF) to ensure VIF < 4 for all fixed factors. I used negative log likelihood (−2LL) to indicate model fit, and I use a likelihood ratio test to compare full models to intercept-only models to test model fit. I set α at 0.05.
I examined the relationship between individual mean glucocorticoid concentrations and mean individual activity variables using Spearman’s rank correlations in the 11 subjects. Because activity variables are not independent of each other, I used a Bonferroni correction to account for multiple testing, with the adjusted α = 0.0125. I presented activity budget variables as mean ± standard deviation.
I conducted all analyses in SPSS (IBM Corp., Armonk, NY), and reported two-tailed P-values for all tests.
Ethical Note
I conducted all research in accordance with the Ohio State University’s Institutional Animal Care and Use Committee protocol #2008A0098, and the regulations of Costa Rica’s Ministerio de Ambient, Energía, y Mares. I conducted all research with the permission of the private landowner, and adhered to the International Primatological Society and American Society of Primatologists ethical guidelines.
Results
Fruit availability varied over the year (Table II). The primary fruits the spider monkeys consumed included Spondias, Hyeronima, Ficus, Musa, and walking palms (Socretea/Iriartea). Fruit abundance was low in September–December (ΣDBH 292.9 ± 30.0 cm), but remained high in January–August (ΣDBH 657.3 ± 34.7 cm; Fig. 1). Subgroup size differed significantly between periods of low fruit abundance and high fruit abundance (low: 2.31 ± 1.27 individuals; high: 3.05 ± 2.01 individuals; U = 70682.00, N = 861, P < 0.001), whereas fecal glucocorticoid metabolite concentrations did not differ significantly between periods of low fruit abundance and high fruit abundance (low: 12.55 ± 2.23 ng/g; high: 15.4 ± 3.28 ng/g; U = 1874.50, N = 134, P = 0.803; Fig. 2).
Fig. 1.
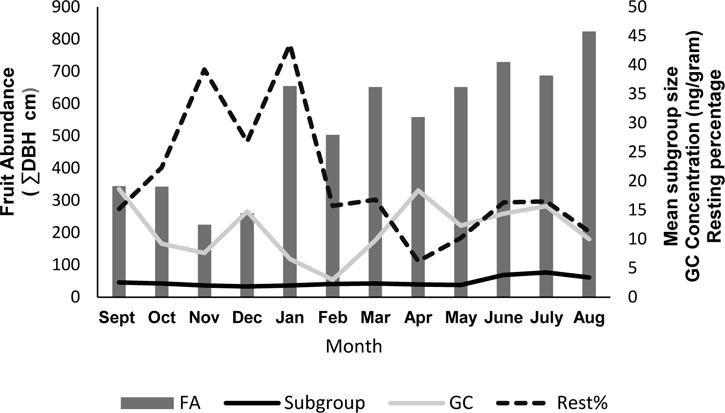
Monthly fruit abundance, mean subgroup size, mean glucocorticoid concentrations (GC), and mean resting percentage for female Geoffroy’s spider monkeys (Ateles geoffroyi) in the secondary forest of El Zota Biological Field Station, Costa Rica, from September 2010 to August 2011.
Fig. 2.

Tukey outlier boxplot of subgroup sizes for female Geoffroy’s spider monkeys (Ateles geoffroyi) at El Zota Biological Field Station during periods of low fruit abundance (September–December) and periods of high fruit abundance (January–August). Boxes represent the interquartile range, and whiskers indicate 1.5× the interquartile range. Medians are indicated with a line. Confidence diamonds indicate the mean and the upper/lower 95% of the mean. Outliers are indicated by black circles, and individual data points are indicated by open gray circles.
The full model for subgroup size significantly predicted subgroup size (F3,68 = 8.671; P < 0.001; −2LL = 95.865), and was a better fit than the null model (intercept-only: −2LL: 251.503; likelihood ratio test: χ2 = 15.975; df = 2, P < 0.001). In the full model for subgroup size, only fruit abundance significantly predicted subgroup size (GLMM: fruit abundance: F1,68 =22.555, P < 0.001; reproductive state: F2,68 = 1.052, P = 0.4355; −2LL = 95.865). Fruit abundance and subgroup size were positively related.
The full model for glucocorticoid concentration significantly predicted subgroup size (F8,63 = 55.138, P < 0.001; −2LL = 236.077), and was a better fit than the null model (intercept-only: −2LL: 541.474; likelihood ratio test: χ2 = 27.080, df = 7, P < 0.001). In the full model for glucocorticoids, rest and reproductive state significantly predicted glucocorticoid concentration (Table III). Glucocorticoids were negatively related to time spent resting, and highest in cycling females.
Table III.
Test of fixed effects for full general linear mixed model (GLMM) for effects of activity budget, fruit abundance, subgroup size, and reproductive state on glucocorticoid metabolite concentration in female Geoffroy’s spider monkeys (Ateles geoffroyi) at El Zota Biological Field Station, Costa Rica, September 2010–August 2011
| Fixed Effect | Numerator df | Denominator df | F | Significance |
|---|---|---|---|---|
| Travel | 1 | 63 | 1.783 | 0.187 |
| Feed | 1 | 63 | 1.894 | 0.174 |
| Rest | 1 | 63 | 5.295 | 0.025* |
| Social | 1 | 63 | 2.425 | 0.124 |
| Fruit abundance | 1 | 63 | 0.277 | 0.600 |
| Subgroup size | 1 | 63 | 0.725 | 0.398 |
| Reproductive state | 2 | 63 | 7.999 | <0.00* |
Asterisks indicate significant effects.
Individuals (N = 11) spent 33.5 ± 4.5% of their time feeding, 23.0 ± 5.5% resting, 29.8 ± 6.1% traveling, 6.2 ± 4.0% socializing, and 7.5 ± 2.1% out of sight or engaged in other behaviors. Individual time engaged in rest and mean fecal glucocorticoid metabolite concentration over the entire year were significantly negatively correlated (Spearman rank correlation: rs = −0.737, P = 0.010; Fig. 3). However, there was no relationship between feeding (rs = 0.260, P = 0.440), travel (rs = 0.474, P = 0.474) or social behavior (rs = 0.014, P = 0.968) and mean fecal glucocorticoid metabolite concentrations.
Fig. 3.

Scatterplot of mean fecal glucocorticoid metabolite concentrations and percent time engaged in rest for individual female Geoffroy’s spider monkeys (Ateles geoffroyi) at El Zota Biological Field Station, June 2010–August 2011.
Discussion
In accordance with my predictions, I found that subgroups were significantly larger when fruit abundance was high and smaller when fruit abundance was low. However, glucocorticoid concentration did not significantly differ between periods of high and low fruit abundance. Contrary to my predictions, fruit abundance was not a significant predictor of glucocorticoid concentration; only resting time and reproductive state significantly predicted glucocorticoid concentration. Furthermore, as predicted, increased resting time was associated lower glucocorticoids, but surprisingly, foraging and traveling were not significantly associated with glucocorticoid concentrations. The significant influence of fruit abundance on subgroup size, but not glucocorticoid concentrations, suggests that fission–fusion dynamics mediate the impact of variation in fruit abundance on spider monkey glucocorticoids. Subgroups were significantly larger during periods of high fruit abundance, and monthly fruit abundance significantly predicted subgroup size. Contrary to my predictions, glucocorticoids did not vary significantly between periods of low and high fruit abundance, and fruit abundance did not predict glucocorticoid concentration. Although fruit abundance, activity patterns, and subgroup size did not predict glucocorticoid concentrations, resting time and reproductive stated significantly predicted glucocorticoid concentrations. Over the year, individual resting time was negatively correlated with individual glucocorticoid concentrations. If resting time is influenced by anthropogenic factors such as habitat disturbance (González-Zamora et al. 2011), this is a potential indirect effect of anthropogenically altered habitat on glucocorticoid concentrations. Although the spider monkeys consumed mostly wild fruit, they also foraged on planted foods such as pílon and bananas. The availability of these resources may alter the impact anthropogenic disturbances such as fragmentation have on monkeys’ physiological stress in this habitat.
Fruit Abundance and Subgroup Size
The positive relationship between fruit abundance and subgroup size is consistent with most other studies of spider monkey grouping patterns, as well as some chimpanzee and bonobo grouping patterns (spider monkeys: Asensio et al. 2008, 2009; Chapman et al. 1995; Schaffner et al. 2012; Symington 1990; chimpanzees and bonobos: Basabose 2004; Chapman et al. 1995; Itoh and Nishida 2007; Matsumoto-Oda et al. 1998; Mulavwa et al. 2008). In most studies of spider monkey grouping patterns, subgroup size fluctuates in response to food availability, with larger subgroups formed when resources are more abundant (Asensio et al. 2008, 2009; Chapman et al. 1995; Symington 1990). Adjustment of subgroup sizes reduces both scramble and contest competition (Asensio et al. 2008, 2009). However, contrary to my predictions that glucocorticoids would rise when fruit abundance was low, fruit abundance was not a significant predictor of glucocorticoid concentrations. My findings suggest that at El Zota, adjustment of subgroup size buffers individuals from stress during periods of low fruit availability. Alternatively, it is possible that even during periods of lower fruit availability, this environment is sufficiently productive to avert ecological stress. However, increases in subgroup size during months with the highest fruit abundance suggest that fruit availability is a limiting factor. My finding is consistent with hypotheses based on fission–fusion dynamics in chimpanzees, which predict that primates adjust subgroup size in relation to fruit abundance when it is a limiting factor (Hashimoto et al. 2003). In Geoffroy’s spider monkeys coping with hurricane-induced disturbance, monkeys range in smaller subgroups to cope with lower fruit availability (Schaffner et al. 2012). Taken together, these results support the hypothesis that high fission–fusion dynamics allow a rapid response to environmental changes that mediates stressors.
Activity Budgets and Glucocorticoids
A combination of ecological, physiological, and social factors affect resting time (Bronikowski and Altmann 1996). The mean time females spent in each activity are comparable to those in a meta-analysis of activity patterns in Geoffroy’s spider monkeys (González-Zamora et al. 2011), as well as a previous study conducted at El Zota (Lindshield 2006). However, resting times for females in my study (23%) are on the lower spectrum of the ranges in the meta-analysis while traveling is higher (30%). In the meta-analysis, resting time was lower in forests with higher rainfall and less seasonality (González-Zamora et al. 2011). Thus, in an environment with mild seasonality and heavy rainfall, resting times should be lower than average. In a previous study at El Zota, females in the primary forest spent more of their time resting than females in the secondary forest (Lindshield 2006). If habitat disturbance influences activity budgets by increasing travel or feeding costs and decreasing available resting time (González-Zamora et al. 2009; Ordóñez-Gómez et al. 2016), this could indicate an indirect cost that the habitat exerts on the monkeys. However, neither travel nor feeding showed a significant relationship with glucocorticoids in this study.
The association between resting time and lower glucocorticoid concentrations reflects findings in another study of Geoffroy’s spider monkeys in Mexico (Ordóñez-Gómez et al. 2016), as well as a study of chacma baboons (Papio hamadryas ursinus: Weingrill et al. 2004). In chacma baboons, extra time available for rest allows animals greater predictability and control over stressors, because animals can preferentially rest during the hottest parts of the day and hide from predators and conspecific aggression (Weingrill et al. 2004). Although spider monkeys at El Zota have lower rates of predation, spider monkeys have a pattern of female-directed coalitionary aggression that is particularly prolonged and stressful for cycling females (Campbell 2003; Rodrigues et al. 2015; Slater et al. 2008). Additionally, resting time may directly affect glucocorticoid metabolism. The definition of baseline glucocorticoid concentration is that of an individual undisturbed at rest (Landys et al. 2006). Animals can achieve adequate resting time only when they are not under foraging or social stress. There are differing hypotheses for activity budget tradeoffs; whereas some hypothesize that animals should reduce social time in response to increased foraging time (Altmann 1980), others argue that they should reduce resting time when foraging time increases (Dunbar and Dunbar 1988). Research on activity trade-offs in yellow baboons (Papio cynocephalus) indicates the amount of time engaged in social behavior is flexible, supporting the hypothesis that social time should be reduced (Bronikowski and Altmann 1996).
Stress in Anthropogenic Habitats
The results of my study at El Zota support some findings from previous studies on stress in spider monkeys living in anthropogenic habitats, but differ from others. Geoffroy’s spider monkeys living in the Lacondona rainforest in Mexico exhibited a negative relationship between resting times and glucocorticoids (Ordóñez-Gómez et al. 2016). However, in that study, decreased travel time and fruit consumption also affected glucocorticoids (Ordóñez-Gómez et al. 2016). Furthermore, they also found that basal area of fruiting trees was negatively associated with glucocorticoids, suggesting that food scarcity was a source of stress (Ordóñez-Gómez et al. 2016). Most importantly, direct anthropogenic stressors such as logging and hunting activity predicted elevated glucocorticoids (Ordóñez-Gómez et al. 2016). Brown spider monkeys in forest fragments at Hacienda San Juan del Carare traveled in smaller subgroups when fruit availability is high, and exhibited higher glucocorticoids (Rimbach et al. 2014). This unexpected pattern, contrary to the findings of most studies on spider monkey subgrouping patterns (Asensio et al. 2008, 2009; Chapman et al. 1995; Schaffner et al. 2012; Symington 1990), is a consequence of increased contest competition (Rimbach et al. 2014). Additionally, the small forest fragment size, high population density, and unexpected intergroup competition exacerbated resource competition. My results also differ from a study of multiple forest types in the Yucatán Peninsula (Rangel-Negrín et al. 2009). Spider monkeys living in large conserved habitats exhibited seasonal elevation of glucocorticoids during the dry season, whereas those in fragmented, anthropogenically disturbed habitat exhibited high glucocorticoids throughout the year (Rangel-Negrín et al. 2009).
The results of my study suggest that in a relatively large, protected habitat, spider monkeys can cope successfully with anthropogenic disturbance, and adjustment of subgroup size in response to fruit availability protects against ecological stresses exerted by habitat fragmentation. However, this is due to a few factors specific to this environment. First, this habitat receives heavy rainfall and only exhibits minor seasonal variation in rainfall (Sanford et al. 1994; Wolfe and Ralph 2009). Thus, the spider monkeys of El Zota do not experience any true periods of resource scarcity, unlike seasonal forests which experience marked dry periods. Second, though there is fragmentation, the overall size of the protected forest area of El Zota is large (1000 ha, supporting two spider monkey communities). Thus, the monkeys here are not affected as strongly as those in the smaller fragments of Hacienda San Juan del Carare in Colombia (65 ha supporting two spider monkey communities: Rimbach et al. 2014), or the fragmented Yucatan sites (<200 ha: Rangel-Negrín et al. 2009). Finally, anthropogenic sources of food alleviate potential foraging stress. Pílon is a native plant species to Costa Rica, and is consumed by spider monkeys at other sites (Luckett et al. 2004; Russo et al. 2005; Suarez 2006). The station owner initially planted it for sustainable harvesting, but then left it untouched to allow reforestation (Luckett et al. 2004). This species was the top food resource in my study. Bananas, which were in plantation left to the wildlife as well as the station garden, were one of the top five food sources. This is surprising, because prior to this study, there was only a single observation of spider monkeys feeding in the banana plantations (pers. obs., cited in Lindshield 2006). Bananas may be particularly important during periods of time where preferred foods such as hogplum, pílon, and figs are not available. Future research quantifying foraging time is necessary to examine the importance of bananas during periods of low fruit availability. Finally, unlike study sites with direct anthropogenic stressors such as hunting and logging, this study site is protected, and thus most potential anthropogenic stressors are those with indirect effects such as habitat disturbance.
Limitations and Future Directions
My conclusions are limited by the scope of the study as well as methods used. Previous research compared activity budgets and ecology (Lindshield 2006), but future research directly comparing glucocorticoids between the secondary and primary forest at El Zota is necessary to strengthen my conclusions. Furthermore, more appropriate methods are necessary to examine the relationship between ecological variables and energetic stress. While glucocorticoids are often used as a proxy for energetic balance, they are confounded by other stressors, such as social stress (Emery Thompson 2016). Direct measurements of energetic balance, including observational measures of food intake and measurement of urinary c-peptide, are necessary to directly test the effects of ecological factors on individual energetic balance (Emery Thompson 2016). Incorporating such measures in conjunction with glucocorticoids will clarify the impact of ecological stressors versus social stressors.
Incorporating a standard scale of anthropogenic disturbance will ease comparison of specific types of anthropogenic pressures (McKinney 2015). Second, the use of comparable methods facilitates comparisons across studies. The methods used here to estimate fruit abundance, quantify activity budget, and assay fecal glucocorticoids are well-established techniques that are easily used across sites (Altmann 1974; Chapman et al. 1992; Ziegler and Wittwer 2005). Meta-analysis of spider monkey activity budget and diet demonstrates the advantages of comparing such data across sites (González-Zamora et al. 2009, 2011). Currently, there are only a few studies on examining spider monkey glucocorticoids in relation to anthropogenic stressors (Ordóñez-Gómez et al. 2016; Rangel-Negrín et al. 2009; Rimbach et al. 2014). Anthropogenically altered habitats may exert mixed effects on monkeys, and collecting data at sites across the continuum of anthropogenic disturbance is necessary to facilitate informed conservation decisions.
Conclusions
Overall, my study indicates that spider monkeys cope with some level of anthropogenic disturbance, and that fission–fusion dynamics facilitate this coping ability. This suggests that although frugivores are vulnerable to disturbance (Sorensen and Fedigan 2000), flexibility in grouping patterns buffers primates with high fission–fusion dynamics from the impact of some anthropogenic disturbances. However, lower resting time predicts higher glucocorticoid concentrations, and previous research at this site indicates that spider monkeys living in the primary forest allot more time to rest (Lindshield 2006). Thus, this is one negative consequence of the disturbed habitat that needs further investigation. Nonetheless, the spider monkeys at this site are less affected by fluctuations in fruit availability and habitat fragmentation compared to other sites. The level and degree of anthropogenic disturbance differs between sites (McKinney 2015) and more research is necessary on how spider monkeys are affected by disturbance. My study indicates that spider monkeys can successfully cope with mildly disturbed habitats, and anthropogenically provided foods are a valuable resource. However, although this is not harmful in a protected site dedicated to research and education, foraging on anthropogenic food sources is risky in contexts where they may encounter human–wildlife conflict. Further research in unprotected areas, particularly in agroecosystems (Estrada 2006) is needed to identify if this behavior occurs at other sites.
Acknowledgments
This research was supported by the Wenner-Gren Foundation, the American Philosophical Society Lewis and Clark Fund, the Ohio State Alumni Grant, and the Ohio State chapter of Sigma Xi. Hormonal assays were partially supported by an NIH grant RR000167 to Toni Ziegler, and performed by Dan Wittwer at the Wisconsin National Primate Center. I thank Hiner Ramirez, his family, and the El Zota staff for their hospitality and Israel Mesen for logistical support. I appreciate the guidance of Dawn Kitchen in all aspects of this project, Jill Pruetz and Erin Ehmke for methodological advice, and Matthew Lattanzio for statistical advice. I am especially grateful to Emily Stulik, Anna Kordek, and Lindsay Mahovetz for their efforts in collecting and processing samples in the field, and Jason Ferrell for assistance in setting up transects. This manuscript benefited from comments from Dawn Kitchen, Scott McGraw, Randy Nelson, Douglas Crews, Stacy Lindshield, Tracie McKinney, Laurie Kauffman, Erin Kane, Cathy Cooke, and Summer Sanford, and was strengthened by suggested revisions from Paul Garber, Joanna Setchell, and several anonymous reviewers.
Footnotes
Data Availability Statement
The data analyzed during the current study are available from the corresponding author upon reasonable request.
References
- Ahumada J. Grooming behavior of spider monkeys (Ateles geoffroyi) on Barro Colorado Island, Panama. International Journal of Primatology. 1992;13:33–49. [Google Scholar]
- Altmann J. Observational study of behavior: Sampling methods. Behavior. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Asensio Ng, Korstjens AH, Aureli F. Fissioning minimizes ranging costs in spider monkeys: A multiple-level approach. Behavioral Ecology Sociobiology. 2009;63:649–659. [Google Scholar]
- Asensio N, Korstjens AH, Schaffner CM, Aureli F. Intragroup aggression, fission–fusion dynamics and feeding competition in spider monkeys. Behaviour. 2008;145:983–1001. [Google Scholar]
- Aureli F, Schaffner C, Boesch C, Bearder S, Call J, et al. Fission-fusion dynamics: New research frameworks. Current Anthropology. 2008;49:627–654. [Google Scholar]
- Baden AL, Webster TH, Kamilar JM. Resource seasonality and reproduction predict fission-fusion dynamics in black-and-white ruffed lemurs (Varecia variegata) American Journal of Primatology. 2016;78:256–279. doi: 10.1002/ajp.22507. [DOI] [PubMed] [Google Scholar]
- Basabose A. Fruit availability and chimpanzee party size at Kahuzi montane forest, Democratic Republic of Congo. Primates. 2004;45:211–219. doi: 10.1007/s10329-004-0087-7. [DOI] [PubMed] [Google Scholar]
- Beehner JC, McCann C. Seasonal and altitudinal effects on glucocorticoid metabolites in a wild primate (Theropithecus gelada) Physiology & Behavior. 2008;95:508–514. doi: 10.1016/j.physbeh.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, et al. Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology and Evolution. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Bronikowski AM, Altmann J. Foraging in a variable environment: Weather patterns and the behavioral ecology of baboons. Behavioral Ecology and Sociobiology. 1996;39:11–25. [Google Scholar]
- Campbell CJ. Doctoral dissertation. University of California Berkeley; 2000. The reproductive biology of black-handed spider monkeys (Ateles geoffroyi): Integrating behavior and endocrinology. [Google Scholar]
- Campbell CJ. Female-directed aggression in free-ranging Ateles geoffroyi. International Journal of Primatology. 2003;24:223–237. [Google Scholar]
- Carnegie SD, Fedigan LM, Ziegler TE. Social and environmental factors affecting fecal glucocorticoids in wild, female white-faced capuchins (Cebus capucinus) American Journal of Primatology. 2011;73:861–869. doi: 10.1002/ajp.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA. Association patterns of spider monkeys: The influence of ecology and sex on social organization. Behavioral Ecology and Sociobiology. 1990;26:409–414. [Google Scholar]
- Chapman CA, Chapman LJ, Wrangham R. Ecological constraints on group size: An analysis of spider monkey and chimpanzee subgroups. Behavioral Ecology and Sociobiology. 1995;36:59–70. [Google Scholar]
- Chapman CA, Chapman LJ, Wrangham R, Hunt K, Gebo D, Gardner L. Estimators of fruit abundance of tropical trees. Biotropica. 1992;24:527–531. [Google Scholar]
- Chapman CA, Wrangham R, Chapman L. Indices of habitat-wide fruit abundance in tropical forest. Biotropica. 1994;26:160–171. [Google Scholar]
- Cuarón A, Morales A, Shedden A, Rodriguez-Luna E, de Grammont P, Cortés-Ortiz L. Ateles geoffroyi. IUCN Red List of Endangered Species. 2008;2008 [Google Scholar]
- Di Fiore A, Campbell C. The Atelines: Variation in ecology, behavior, and social organization. In: Campbell C, Fuentes A, MacKinnon K, Panger M, Bearder S, editors. Primates in perspective. Oxford; Oxford University Press; 2007. pp. 155–185. [Google Scholar]
- Dolado R, Cooke C, Beltran FS. How many for lunch today? Seasonal fission-fusion dynamics as a feeding strategy in wild red-capped mangabeys (Cercocebus torquatus) Folia Primatologia. 2016;87:197–212. doi: 10.1159/000449220. [DOI] [PubMed] [Google Scholar]
- Dunbar R, Dunbar P. Maternal time budgets of gelada baboons. Animal Behavior. 1988;36:970–980. [Google Scholar]
- Emery Thompson M. Energetics of feeding, social behavior, and life history in nonhuman primates. Hormones and Behavior. 2016 doi: 10.1016/j.yhbeh.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Estrada A. Human and non-human primate co-existence in the Neotropics: A preliminary view of some agricultural practices as a complement for primate conservation. Ecological and Enhvironmental Anthropology. 2006;2:17–29. [Google Scholar]
- Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, et al. Impending extinction crisis of the world’s primates: Why primates matter. Science Advances. 2017;3:e1600946. doi: 10.1126/sciadv.1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster S, Cords M, Monfort SL. Seasonal energetic stress in a tropical forest primate: Proximate causes and evolutionary implications. PLoS ONE. 2012;7(11):e50108. doi: 10.1371/journal.pone.0050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulin SJC, Sailer LD. Are females the ecological sex? American Anthropologist. 1985;87:111–119. [Google Scholar]
- Gentry A. Patterns of Neotropical plant-species diversity. Evolutionary Biology. 1982;15:1–85. [Google Scholar]
- Gesquiere LR, Khan M, Shek L, Wango TL, Wango EO, et al. Coping with a challenging environment: Effects of seasonal variability and reproductive status on glucocorticoid concentrations of female baboons (Papio cynocephalus) Hormones and Behavior. 2008;54:410–416. doi: 10.1016/j.yhbeh.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Buttoz C, Heistermann M, Krummel S, Engelhardt A. Seasonal and social influences on fecal androgen and glucocorticoid excretion in wild male long-tailed macaques (Macaca fascicularis) Physiology and Behavior. 2009;98:168–175. doi: 10.1016/j.physbeh.2009.05.005. [DOI] [PubMed] [Google Scholar]
- González-Zamora A, Arroyo-Rodríguez V, Chaves OM, Sánchez-López S, Aureli F, Stoner KE. Influence of climatic variables, forest type, and condition on activity patterns of Geoffroyi’s spider monkeys throughout Mesoamerica. American Journal of Primatology. 2011;73:1189–1198. doi: 10.1002/ajp.20989. [DOI] [PubMed] [Google Scholar]
- González-Zamora A, Arroyo-Rodríguez V, Chaves OM, Sánchez-López S, Stoner KE, Riba-Hernández P. Diet of spider monkeys (Ateles geoffroyi) in Mesoamerica: Current knowledge and future directions. American Journal of Primatology. 2009;71:8–20. doi: 10.1002/ajp.20625. [DOI] [PubMed] [Google Scholar]
- Hartwell KS, Notman H, Bonenfant C, Pavelka MSM. Assessing the occurrence of sexual segregation in spider monkeys (Ateles geoffroyi yucatanensis), its mechanisms and function. International Journal of Primatology. 2014;35:425–444. [Google Scholar]
- Hashimoto C, Suzuki S, Takenoshita Y, Yamagiwa J, Basabose AK, Furuichi T. How fruit abundance affects the chimpanzee party size: A comparison between four study sites. Primates. 2003:77–81. doi: 10.1007/s10329-002-0026-4. [DOI] [PubMed] [Google Scholar]
- Hockings KJ, Anderson JR, Matsuzawa T. Socioecological adaptations by chimpanzees, Pan troglodytes verus, inhabiting an anthropogenically impacted habitat. Animal Behaviour. 2012;83:801–810. [Google Scholar]
- Itoh N, Nishida T. Chimpanzee grouping patterns and food availability in Mahale Mountains National Park, Tanzania. Primates. 2007;48:87–96. doi: 10.1007/s10329-006-0031-0. [DOI] [PubMed] [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. General and Comparative Endocrinology. 2006;148:132–149. doi: 10.1016/j.ygcen.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Korstjens AH, Dunbar RIM. Fission-fusion social systems as a strategy for coping with ecological constraints: A primate case. Evolutionary Ecology. 2007;21:613–634. [Google Scholar]
- Lindshield SM. Master’s thesis. Iowa State University; 2006. The density and distribution of Ateles geoffroyi in a mosaic landscape at El Zota Biological Field Station, Costa Rica. [Google Scholar]
- Luckett J, Danforth E, Linsenbardt K, Pruetz J. Planted trees as corridors for primates at El Zota Biological Field Station, Costa Rica. Neotropical Primates. 2004;12:6–8. [Google Scholar]
- Martínez-Mota R, Valdespino C, Sánchez-Ramos MA, Serio-Silva JC. Effects of forest fragmentation on the physiological stress response of black howler monkeys. Animal Conservation. 2007;10:374–379. [Google Scholar]
- Matsumoto-Oda A, Hosaka K, Huffman MA. Factors affecting party size in chimpanzees of the Mahale Mountains. International Journal of Primatology. 1998;19:999–1011. [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McKinney T. The effects of provisioning and crop-raiding on the diet and foraging activities of human-commensal white-faced capuchins (Cebus capucinus) American Journal of Primatology. 2011;73:439–448. doi: 10.1002/ajp.20919. [DOI] [PubMed] [Google Scholar]
- McKinney T. A classification system for describing anthropogenic influence on nonhuman primate populations. American Journal of Primatology. 2015;77:715–726. doi: 10.1002/ajp.22395. [DOI] [PubMed] [Google Scholar]
- Moscovice LR, Issa MH, Petrzelkova KJ, Keuler NS, Snowdon CT, et al. Fruit availability, chimpanzee diet, and grouping patterns on Rubondo Island, Tanzania. American Journal of Primatology. 2007;69:487–502. doi: 10.1002/ajp.20350. [DOI] [PubMed] [Google Scholar]
- Mulavwa M, Furuichi T, Yangozene K, Motema-salo B, Idani G, et al. Seasonal changes in fruit production and party size of bonobos at Wamba. In: Furuichi T, Thompson J, editors. The Bonobos: Behavior, ecology & conservation. New York: Springer Science+Business Media; 2008. pp. 121–135. (Developments in Primatology: Progress and Prospects). [Google Scholar]
- Nelson RJ, Drazen DL. Seasonal changes in stress responses. In: Fink G, editor. Encylopedia of stress. 2nd. Vol. 3. San Diego: Academic Press; 2007. pp. 402–408. [Google Scholar]
- Ordóñez-Gómez JD, Cristóbal-Azkarate J, Arroyo-Rodríguez V, Santillán-Doherty AM, Valdez RA, Romano MC. Proximal and distal predictors of the spider monkey’s stress levels in fragmented landscapes. PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0149671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruetz J. The socioecology of adult female patas monkeys and vervets in Kenya. Upper Saddle River, NJ: Pearson Prentice Hall; 2009. [Google Scholar]
- Pruetz J, LaDuke T. New field site: Preliminary census of primates at El Zota Biological Field Station, Costa Rica. Neotropical Primates. 2001;9:22–23. [Google Scholar]
- Rangel-Negrín A, Alfaro J, Valdez R, Romano M, Serio-Silva J. Stress in Yucatan spider monkeys: Effects of environmental conditions on fecal cortisol levels in wild and captive populations. Animal Conservation. 2009;12:496–502. [Google Scholar]
- Rimbach R, Link A, Montes-Rojas A, Di Fiore A, Heistermann M, Heymann EW. Behavioral and physiological responses to fruit availability of spider monkeys ranging in a small forest fragment. American Journal of Primatology. 2014;76:1049–1061. doi: 10.1002/ajp.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA. Master’s thesis. Iowa State University; 2007. Sex differences in the social behavior of juvenile spider monkeys (Ateles geoffroyi) [Google Scholar]
- Rodrigues MA. Emergence of sex-segregated behavior and association patterns in juvenile spider monkeys. Neotropical Primates. 2014;21:183–188. [Google Scholar]
- Rodrigues MA, Wittwer D, Kitchen DM. Measuring stress responses in female Geoffroy’s spider monkeys: Validation and the influence of reproductive state. American Journal of Primatology. 2015;77:925–935. doi: 10.1002/ajp.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SE, Campbell CJ, Dew JL, Stevenson PR, Suarez SA. A multi-forest comparison of dietary preferences and seed dispersal by Ateles spp. International Journal of Primatology. 2005;26:1017–1037. [Google Scholar]
- Sanford R, Paaby P, Luvall J, Phillips E. Climate, geomorphology, and aquatic systems. In: McDade L, Bawa K, Hespenheide H, Hartshorn G, editors. La Selva: Ecology and natural history of a tropical rainforest. Chicago: University of Chicago Press; 1994. pp. 19–33. [Google Scholar]
- Sapolsky R. Neuroendocrinology of the stress-response. In: Becker J, Breedlove S, Crews D, editors. Behavioral endocrinology. 1st. Cambridge, MA: MIT Press; 1992. pp. 287–324. [Google Scholar]
- Schaffner CM, Rebecchini L, Ramos-Fernandez G, Vick LG, Aureli F. Spider monkeys (Ateles geoffroyi yucatenensis) cope with the negative consequences of hurricanes through changes in diet, activity budget, and fission–fusion dynamics. International Journal of Primatology. 2012;33:922–936. [Google Scholar]
- Shimooka Y. Seasonal variation in association patterns of wild spider monkeys (Ateles belzebuth belzebuth) at La Macarena, Colombia. Primates. 2003;44:83–90. doi: 10.1007/s10329-002-0028-2. [DOI] [PubMed] [Google Scholar]
- Slater KY, Schaffner CM, Aureli F. Female-directed male aggression in wild Ateles geoffroyi yucatanensis. International Journal of Primatology. 2008;29:1657–1669. [Google Scholar]
- Slater KY, Schaffner CM, Aureli F. Sex differences in the social behavior of wild spider monkeys (Ateles geoffroyi yucatanensis) American Journal of Primatology. 2009;71:21–29. doi: 10.1002/ajp.20618. [DOI] [PubMed] [Google Scholar]
- Sorensen TC, Fedigan LM. Distribution of three monkey species along a gradient of regenerating tropical dry forest. Biological Conservation. 2000;92:227–240. [Google Scholar]
- Suarez SA. Diet and travel costs for spider monkeys in a nonseasonal, hyperdiverse environment. International Journal of Primatology. 2006;27:411–436. [Google Scholar]
- Symington MM. Fission-fusion social organization in Ateles and Pan. International Journal of Primatology. 1990;11:47–61. [Google Scholar]
- Trivers R. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. 1871–1971. Chicago: Aldine; 1972. pp. 136–179. [Google Scholar]
- Wakefield ML. Grouping patterns and competition among female Pan troglodytes schweinfurthii at Ngogo, Kibale National Park, Uganda. International Journal of Primatology. 2008;29:907–929. [Google Scholar]
- Weghorst JA. High population density of black-handed spider monkeys (Ateles geoffroyi) in Costa Rican lowland wet forest. Primates. 2007;48:108–116. doi: 10.1007/s10329-006-0025-y. [DOI] [PubMed] [Google Scholar]
- Weingrill T, Gray DA, Barrett L, Henzi SP. Fecal cortisol levels in free-ranging female chacma baboons: Relationship to dominance, reproductive state and environmental factors. Hormones and Behavior. 2004;45:259–269. doi: 10.1016/j.yhbeh.2003.12.004. [DOI] [PubMed] [Google Scholar]
- White FJ. Seasonality and socioecology: The importance of variation in fruit abundance to bonobo sociality. International Journal of Primatology. 1998;19:1013–1028. [Google Scholar]
- Wilson ML, Boesch C, Fruth B, Furuichi T, Gilby IC, et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513:414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- Wolfe JD, Ralph CJ. Correlations between El Niño–Southern Oscillation and changes in Nearctic–Neotropic migrant condition in Central America. Auk. 2009;126:809–814. [Google Scholar]
- Wrangham R. An ecological model of female-bonded primates. Behaviour. 1980;75:262–300. [Google Scholar]
- Ziegler TE, Wittwer DJ. Fecal steroid research in the field and laboratory: Improved methods for storage, transport, processing, and analysis. American Journal of Primatology. 2005;67:159–174. doi: 10.1002/ajp.20175. [DOI] [PubMed] [Google Scholar]


