Figure 4.
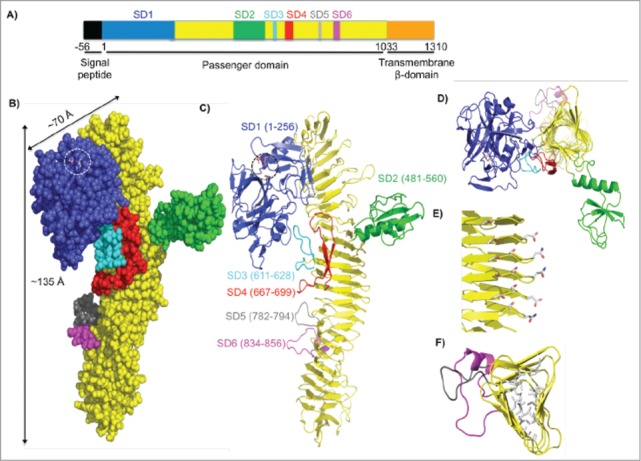
Crystal structure of the SepA passenger domain. (A) Linear schematic representation of the SepA gene. The first 56 residues indicate a signal sequence (black), which directs SepA for secretion. Residues 1–1033 comprise the passenger domain. SD1 contains the serine protease domain (blue, residues 1–256), followed by a β-helix with insertions (yellow, residues 257–1003) including SD2 (green, residues 481–560), SD3 (cyan, residues 611–628), SD4 (red, residues 667–699), SD5 (gray, residues 782–794), and SD6 (magenta, residues 834–856). The C-terminal transmembrane β–domain (orange, residues 1034–1310) folds into a pore from which the passenger domain is secreted. (B) Space-filling representation of the passenger domain using the same color scheme as in the schematic representation of SepA in A. Length and width dimensions are indicated. The Ser211-His78-Asp106 catalytic triad is highlighted and shown as a stick representation. (C) Ribbon diagram representation of the passenger domain. (D) Top view of the passenger domain. For simplicity, residues 257–480 were omitted. Representative sub-domains stem from the corners of the β–helix. (E) Amino acid stacking interactions (Thr-Ser, Ser-Ser, and Asp-Asn; residue numbers are given in Figure S2). (F) Hydrophobic amino acid stacking on the interior of the β-helix as seen through residues 700–900.
