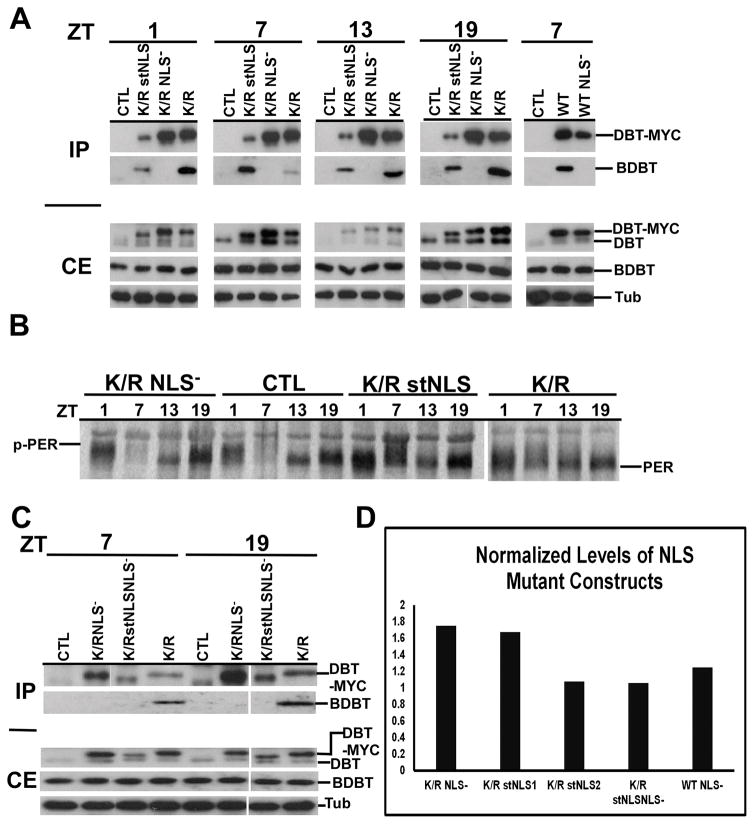
Figure 4.
Immunoblot analysis of head extracts for DBT-MYC demonstrates comparable overall levels of expression of different transgenic DBTs, a lack of interaction between DBTs carrying the NLS mutation and BDBT, and different effects on PER protein oscillations.
(A) Immunoblot analysis of fly head extracts for DBT. The F1 progeny with the indicated genotype of timGAL4/+>UAS-dbt-myc/+ were collected for different mutant genotypes, head extracts were collected and immunoprecipitated with anti-MYC antibody, and blots of SDS PAGE were probed to detect DBT and BDBT levels in the crude extracts (CE) and the immunoprecipitates (IP). DBT was detected using a 1:2000 dilution of anti-DBT-C, which detects both endogenous DBT (lower band) and transgenic DBT-MYC (upper band). The CE blot was then stripped and reprobed with anti-tubulin (lower panel). No clear genotype-specific or circadian changes in DBT-MYC level were seen. Both DBTWT and DBTK/R proteins carrying the NLS mutation failed to interact with BDBT. (B) Immunoblot analysis of fly head extracts for PER. Fly heads were collected for different mutant genotypes at the indicated time points (ZT1: one hour after lights on; ZT7: 7 hour after lights on; ZT13: one hour after lights off; ZT19: 7 hour after lights off) and the blot was probed for PER using a 1:25000 dilution of anti-PER. dbtK/R and dbtK/R stNLS flies produce high levels of hypophosphorylated PER at all four time points, while dbtK/R NLS− flies produce a wild type PER rhythm, compared with wild type flies that inherited a TM3 balancer chromosome instead of a chromosome with the UAS responder gene (CTRL). C) Head extracts of the indicated genotypes were analyzed for DBT expression with an anti-DBT antibody at the indicated circadian times, and they were immunoprecipitated with anti-MYC for detection of DBT and co-immunoprecipitating BDBT. DBTK/R stNLS NLS− protein carries both the NLS− and stNLS mutations, is expressed at comparable levels to the other transgenic DBTs, and like DBTK/R -NLS− and DBTWT NLS− it does not co-immunoprecipitate BDBT. (D) The levels of DBT-MYC expression were assessed for representative lines of each genotype at ZT7 and were found to be equivalent within the range of the measurements. The DBT-MYC signal was quantified for each lane on a gel and normalized to the level of tubulin signal in that lane. Then, this signal was further normalized to the level of DBTK/R/tubulin on the blot for DBTK/R proteins or to DBTWT/tubulin for the DBTWT NLS- construct. Relative signals varied between 1 and 1.8-fold of the DBTK/R or DBTWT baseline signal, and the differences were not statistically significant by one-way ANOVA [F(4,6)=2.8, p>0.1]. Two dbtK/R stNLS lines were tested – lines 2MB (line 1) and line 1MB (line 2). The difference in measured DBT level runs counter to the difference in their average periods (Table 1), but neither of these differences is statistically significant.