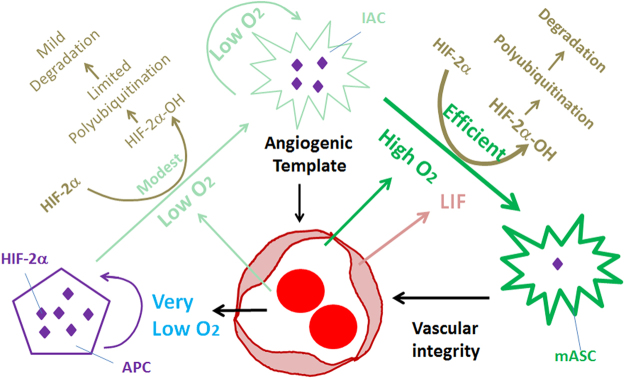
Figure 11.
Interdependence between retinal astrocytic and vascular development in neonatal mice. APCs may be severely hypoxic due to their remote locations from vascularized areas. Hypoxia favors the accumulation of HIF-2α (depicted as many purple diamonds), which in turn promotes APC status32. IACs may be under varying degrees of hypoxia depending on their distances from vascularized areas, but the extent of hypoxia may be generally lighter than in APCs (except in a small number of IACs located more peripherally than APCs). Intermediate levels of oxygen may permit HIF-2α hydroxylation at modest efficiencies. Ongoing synthesis and degradation may result in a steady state level of HIF-2α that is lower than in APCs but higher than in mASCs (represented by the relative numbers of purple diamonds). mASCs are well oxygenated by being within vascularized areas. High levels of oxygen promote efficient HIF-2α prolyl hydroxylation and fast degradation. However, mASCs probably still retain trace amounts of HIF-2α which are functionally important (depicted as a single purple diamond). This possibility is supported by the fact that Hif-2α knockout in differentiated astrocytes led to compromised retinal vascular stability46. Besides oxygen, LIF is also known to induce astrocytic differentiation from immature astrocytes11,34,44. With regard to angiogenesis, IACs but probably not APCs are critical, due to the remote distances of APCs from the vascular front. The responsible angiogenic factor(s) are unknown, but IACs might facilitate angiogenesis by providing a physical surface for endothelial cells to adhere and migrate over7.