Fig 2. MiT/TFE transcription factors control mTORC1 activity through RagD.
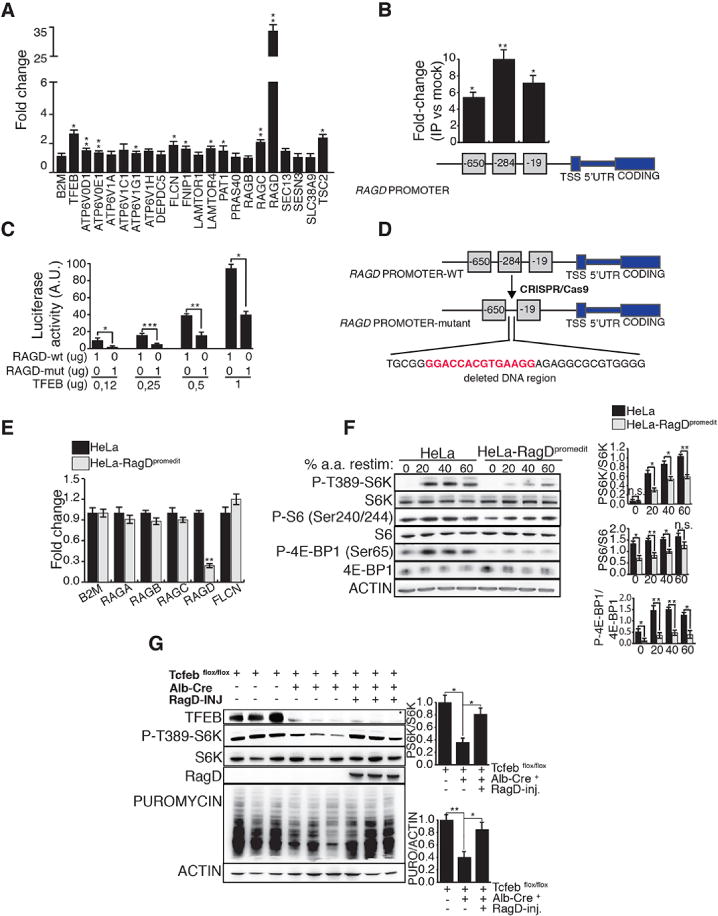
(A) mRNA levels of mTORC1-related genes in TFEB-CA HeLa cells treated with doxycycline. Values were normalized relative to HPRT1 and expressed as fold change relative to untreated cells. (B) ChIP analysis of TFEB binding to RagD promoter in doxycycline treated HeLa TFEB-CA cells. Squares represent CLEAR sites in RagD promoter and numbers refer to their distance (bp) from the transcriptional start site (TSS). Immuno-precipitated DNA was normalized to the input and plotted as relative enrichment over a mock control. (C) Luciferase assay analysis after transfection of increasing amounts of TFEB construct was performed in HeLa cells co-transfected with a wild type (RAGD-wt) or mutated (RAGD-mut) RagD-promoter luciferase reporter plasmids. (D) Scheme of CRISPR/Cas9-mediated mutation in the endogenous RagD promoter of HeLa cells. A region of 33bp containing the CLEAR site at position −284 (in red) was ablated. (E) Transcript levels of Rags and Flcn genes were analyzed in the mutated HeLa cell line (HeLa-RagDpromedit) versus control HeLa and normalized relative to HPRT1 gene. (F) Immuno-blotting analysis of mTORC1 signaling in HeLa-RagDpromedit cells compared to control HeLa. The ratio of phosphorylated/total protein levels were shown for the indicated mTORC1 substrates. The plots in (A), (B), (C), (E), (F) represent mean ± SEM of 3 independent experiments (Student t test). (G) Mice with indicated genotypes were nutritionally synchronized and injected with puromycin 30 minutes prior sacrifice. Where indicated Tcfebflox/flox; Alb-Cre+ mice were injected with an AAV-vector carrying human RagD cDNA. Liver lysates were analyzed for phosphorylation of S6K and levels of puromycin incorporation. The plots show means of triplicates +/− SEM, Anova (one-way) expressed as ratio of phosphorylated S6K/pan-S6K and puromycin/actin. In (A), (B), (C), (E), (F), (G) *p <0.05, **p <0.01, ***p<0.001.
