Abstract
An isolated black yeast-like strain was obtained from radiation-polluted soil collected from Xinjiang province in northwest China. On the basis of ITS and LSU rDNA sequence analysis, in combination with the colony morphology and phenotypic properties, the isolated strain was revealed to represent a novel variety of Aureobasidium subglaciale, designated as A. subglaciale F134. Compared to other yeasts and bacteria, this isolate displayed superior resistance to gamma irradiation, UV light, and heavy metal ions. It was discovered that the resistance of the isolate was correlated with the stress protector trehalose. Through the overexpression of the trehalose-6-phosphate synthase gene tps1 and the deletion of acid trehalase gene ath1, the APT∆A double mutant exhibited a survival rate of 1% under 20 kGy of gamma-radiation, 2% survival rate at a UV dosage of 250 J/m2, and tolerance towards Pb2+ as high as 1500 mg/L, which was in agreement with the high accumulation of intracellular trehalose compared to the wild-type strain. Finally, the protective effects and the mechanism of trehalose accumulation in A. subglaciale F134 were investigated, revealing a significant activation of the expression of many of the stress tolerance genes, offering new perspectives on the adaptations of radioresistant microorganisms.
Introduction
The production and testing of nuclear weapons will release radioactive waste into the environment, which are now contaminating the ground and subsurface at thousands of sites. In China, the largest nuclear test to date was conducted on 21 May 1992 in the Kuruktag and Kyzyltag mountains of Xinjiang Uyghur Autonomous Region, with pollution covering large areas of the surface and underground. These highly toxic waste sites become the ideal places to search for radiation resistant microorganisms1. Recently, various radiation resistant organisms have been isolated by our group from the radiation-contaminated soils in the Xinjiang Uyghur Autonomous Region of Northwest China, belonging to the domains bacteria, archaea and fungi, and several of their stress-resistance genes have been identified by genome sequencing2–7. In the existing nuclear pollution areas, such as the Chernobyl Atomic Energy Station (ChAES), 37 species belonging to 19 genera in the eucarya domain had been successfully discovered before 20008, which was expanded into 200 species from 98 genera in 20049. However, there is an even richer biodiversity at the Xinjiang nuclear explosion area due to adaptation and evolution for more than 50 years10.
Trehalose is a naturally stable, non-reducing disaccharide sugar, in which two glucose units are linked via an α,α-(1,1)-glycosidic bond11. It has been isolated from a large number of prokaryotic and eukaryotic species spanning bacteria, archaea, yeast, fungi, algae, plant, as well as low orders of the animal kingdom, especially those living in extreme environments12. So far, five different enzymatic pathways related to the biosynthesis of trehalose have been uncovered and identified in naturally occurring microorganisms13. Yeast-like cells, mycelia and melanin-pigmented chlamydospores of genus Aureobasidium are particularly known for their biotechnological significance, since this organism is a producer of the extracellular polysaccharide (EPS) pullulan (poly-α-1, 6-maltotriose)14–16. As the type species, Aureobasidium pullulans is an ubiquitous oligotroph that can be found in various environments such as hypersaline waters in salterns17, on rocks and monuments18, as airborne spores19, in food and feeds20, in deserts21, and in industrial effluents22. As previously reported, one copy of the trehalose biosynthesis gene encoding trehalose-6-phosphate synthase (TPS) was found in four investigated A. pullulans varieties, and another relatively dissimilar trehalose-related hydrolase (e.g., trehalase, EC 3.2.1.28) was also identified, which hydrolyzes trehalose into glucose15,23. In addition, the concentration of trehalose in A. pullulans increased when the cells were exposed to high salt concentrations or high temperatures24. However, the biological effects and protective mechanisms of trehalose accumulation in A. pullulans cells exposed to irradiation and heavy metal stress remain unknown.
In this research, a new black yeast-like fungus was isolated from radiation and heavy metal-polluted soil samples from Xinjiang, Uyghur Autonomous Region of Northwest China. Based on ITS and LSU rDNA sequence analysis, the isolated strain belongs to the genus Aureobasidium and represents a novel isolate of A. subglaciale. Furthermore, we generated a trehalose-overproducing mutant strain of A. subglaciale F134 by overexpressing tps1 and disrupting ath1. The mutant strain showed a 3-fold increase in biosynthesis of trehalose, and furthermore displayed enhanced resistance characteristics, confirming that there is an interplay between trehalose metabolism and the oxidative stress response in Aureobasidium.
Results and Discussion
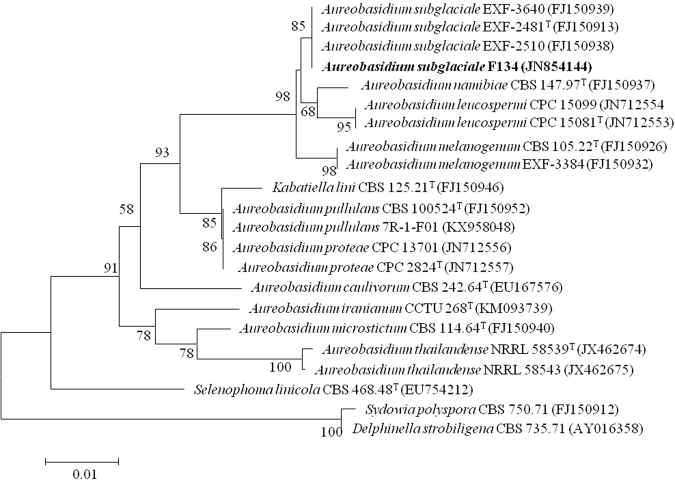
Strain isolation and phylogenetic analyses
A total of 13 isolates comprising 6 strains of mycelial fungi and 7 strains of yeast from the polluted soil samples were obtained on Czapek25 and potato dextrose agar (PDA)26 plates containing different concentration of Pb2+. As a result, only one isolated yeast-like strain grew well in the presence of 0.12% Pb2+ on PDA plates, and the morphological analysis indicated that this isolate was highly likely to be a member of the genus Aureobasidium (Figures S1, S2)27. According to sequence comparisons of the internal transcribed spacer (ITS) region and the D1/D2 domains of the large-subunit (LSU) rRNA gene, the isolated strain was preliminarily identified as a novel yeast strain of the genus Aureobasidium. A phylogenetic tree study was further performed with the LSU rRNA gene sequences of type strains of all the species from the genus Aureobasidium (Fig. 1). According to the similarity matrix based on the alignment of all cited representative LSU rRNA gene sequences, a high level of support indicated that the strain was closely related to A. subglaciale, which is member of the secondary clade and shared a 85% homology with A. subglaciale EXF-2510 (FJ150938), A. subglaciale EXF-2481T (FJ150913) and A. subglaciale EXF-3640 (FJ150939). Based on above results, we proposed to name this isolated strain of Aureobasidium as A. subglaciale F134, which could be readily distinguished from the type strain of A. pullulans 16 by a combination of phenotypic properties (Table S1).
Figure 1.
Phylogenetic tree, reconstructed based on the D1/D2 domain of LSU rRNA gene by using the neighbor-joining method implemented with MEGA version 7.0, showing that the strain A. subglaciale F134 had a great similarity with relevant species in the geuns Aureobasidium. Bootstrap values >50% were shown on the branches and bootstrap values were set at 1000 iterations. The scale bar was considered as an evolutionary distance of 0.01 nucleotide substitution rates.
Trehalose metabolism in A. pullulans
The most widespread trehalose pathway is OtsA-OtsB, which is found in all the prokaryotic and eukaryotic organisms12. This pathway starts when trehalose-6-phosphate synthase (TPS) catalyzes the transfer of glucose from UDP-glucose to glucose-6-phosphate (G-6-P) with the formation of trehalose-6-phosphate (T-6-P) and uridine diphosphate (UDP). The T-6-P is therewith dephosphorylated into trehalose by trehalose-6-phosphate phosphatase (TPP). In additon, trehalose can be hydrolyzed to glucose by either a cytosolic neutral trehalases (NTH) or a vacuolar acidic trehalase (ATH), which may bring the levels of trehalose back to normal equilibrium levels once the stress is over28. Previous studies have successfully engineered yeast strains through the overexpression or deletion of trehalose metabolic genes (tps1, tps2, tps3, tsl1, ath1, nth1, and nth2), with the aim to investigate the protective function of trehalose under various stress conditions28,29. Importantly, the availability of genome sequence data for A. pullulans EXF-150 and A. pullulans AY4 made it possible to screen the new isolate from this study for the presence of ORFs sharing homology with genes related to trehalose metabolism16,30. The ORFs with high similarity to apparent orthologs from three model microorganisms involved in the synthesis and degradation of trehalose were found in A. subglaciale F134 and the results are summarized in Table 1. As can be seen in Table 1, a BLAST search mainly based on the known Saccharomyces cerevisiae pathway genes identified ORFs with putative functions as a homologue of Tps1 and Ath1 as having 69% and 32% overall identity respectively, at the amino acid level. As a result, the most highly expressed genes tps1 and ath1 were chosen for engineering by overexpression and deletion, respectively, with the aim to improve intercellular trehalose accumulation.
Table 1.
Homologues of trehalose biosynthesis enzymes and their identity in A. subglaciale F134.
| Matching sequences | |||||
|---|---|---|---|---|---|
| A. subglaciale ORFs | Length (aa) | strain | Description | Length (aa) | Identity (%) |
| Tps1 (putative) | 541 | Saccharomyces cerevisiae | trehalose 6-phosphate synthase Tps1 | 495 | 69% |
| Arabidopsis thaliana | trehalose 6-phosphate synthase Tps1 | 942 | 49% | ||
| Escherichia coli | cytoplasmic trehalase | 549 | 36% | ||
| Ath1 (putative) | 792 | Saccharomyces cerevisiae | alpha, alpha- trehalase ath1 | 1211 | 32% |
| Arabidopsis thaliana | vacuolar acid trehalase | 473 | 31% | ||
| Escherichia coli | trehalose-6-P hydrolase | 551 | 78% | ||
In order to demonstrate the effects of genetic manipulation, the enzyme activities of TPS and ATH as well as the intercellular trehalose contents of the wild-type strain AP and its engineered derivatives were compared. As expected, the engineered strain APtps1 had higher TPS activity, strain AP∆ath1 had lower ATH activity, and strain APT∆A had a higher TPS and a lower ATH activity, compared with the wild-type strain (Fig. 2). Therefore, the three engineered strains all showed a significant increase of intracellular trehalose levels compared with their wild-type parent strain (Table 2). Among these engineered strains, the strain APT∆A exhibited a 3-fold increase in trehalose content without stress induction (39.3 ± 1.9 vs. 13.1 ± 0.6 mg trehalose/g dry cell weight) and even more in the presence of Pb2+ (212.3 ± 10.8 vs. 62.1 ± 3.1 mg trehalose/g dry cell weight), which demonstrated the significant effect on trehalose content that can be achieved by the overexpression of TPS1 combined with deletion of ATH1 in A. pullulans.
Figure 2.
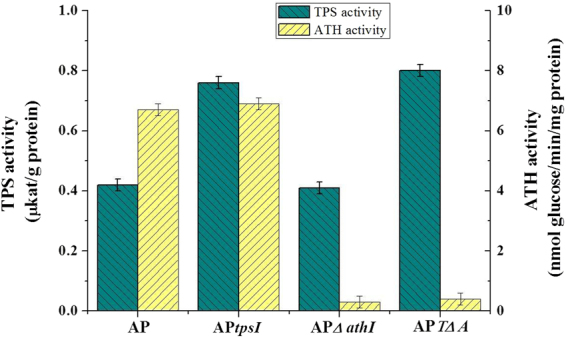
The enzyme activities of TPS and ATH between wild strain AP and its engineered strains APtps1, AP∆ath1 and APT∆A were compared. Each error bar indicates +/− one standard error of the mean.
Table 2.
Intracellular trehalose levels of A. subglaciale F134 wild-type and trehalose biosynthesis mutants under different conditionsa.
| Strains | Trehalose yield (mg/g dry weight) | |
|---|---|---|
| YPD | Pb2+ (1,000 mg/L) with YPD | |
| AP | 13.1 ± 0.55 | 62.1 ± 3.12 |
| APtps1 | 25.8 ± 2.24 | 110.7 ± 5.49 |
| AP∆ath1 | 19.5 ± 0.99 | 98.2 ± 3.75 |
| APT∆A | 39.3 ± 1.87 | 212.3 ± 10.8 |
| Pb | 0.00 | 0.00 |
aStrains were precultured in YPD medium to stationary-phase, and cells were collected and cultured in YPD with or without the stress factors at 28 °C with 200 rpm for 10 h. All data shown are mean values from at least three replicate experiments.
bP < 0.05 indicates there are statistically significant differences between the four strains.
Resistance to radiation and heavy metals
Stress tolerance is often associated with accumulation of small organic molecules, which can act as protective compounds against various stress factors31. In yeasts and other microorganisms, a large body of research suggests that trehalose serves not only as carbohydrate storage molecule, but also as a metabolic regulator and protective agent against a wide variety of abiotic stresses, such as heat32, cold33, dehydration34, desiccation35, and oxygen radicals36. Therefore, we examined the growth characteristics of the wild-type AP and the mutant strain APT∆A under radiation and heavy-metal stress conditions.
Gamma-radiation and UV-light survival curves for both the wild-type and the mutant strain APT∆A showed a characteristic shoulder form, as opposed to the nearly linear inactivation kinetics observed for Escherichia coli (Fig. 3). As can be seen in Fig. 3, there was a decrease in the number of CFU/g recovered from the wild-type AP strain with increasing doses of gamma rays and increasing irradiation time with UV light. Significantly, this lethality trend was slowed significantly in the mutant strain APT∆A. For example, after exposure to 20 kGy, less than 0.1% of the original culturable AP cells could be recovered by dilution plating, while the surviving fraction was 10-times larger, at nearly 1%, in the mutant strain APT∆A. Even so, the genus Aureobasidium is still resistant to levels of gamma radiation that far exceed the background levels in the natural environment, and was obviously superior to Phaffia rhodozyma with a survival rate of 0.1% under a lower dosage of 3 kGy37, and Saccharomyces cerevisiae with a survival rate of less than 0.01% under 150 J/m2 38. Moreover, compared with the well-known radiation-resistant bacterium Deinococcus radiodurans R1, the survival rates of both the wild-type and the mutant strain APT∆A were in the equivalent order of magnitude when exposed to the same does of gamma and UV radiation, including an extraordinary performance at UV does as high as 250 J/m2 (Fig. 3), which makes them compare favorably with the world’s most recognized radioresistant microorganisms39.
Figure 3.
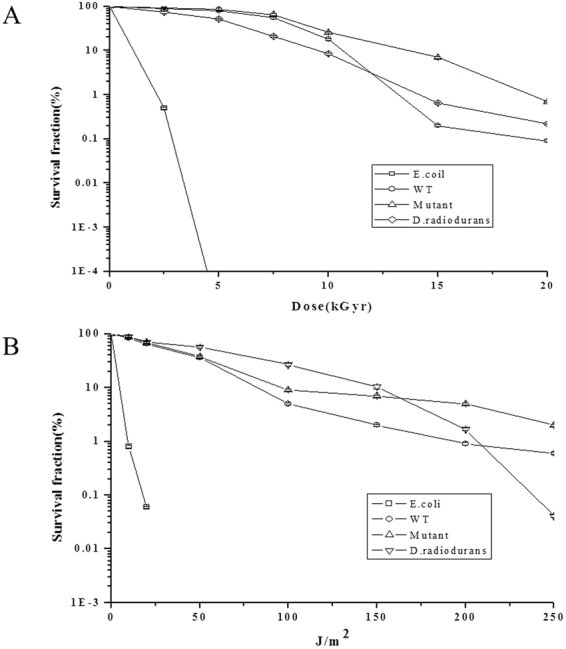
Survival fraction of the isolates exposed to gamma and UV rays. (A) Doses of gamma rays. (B) Doses of UV rays. The isolates of Aureobasidium strain wild-type AP and modified mutant strain APT∆A were test with E. coli as a negative contrast and D. radiodurans as a positive contrast. Each error bar indicates the average value obtained in the three experiments.
Additionally, the resistance of both the wild-type AP and the mutant strain APT∆A to different heavy metals was also investigated (Fig. 4). Members of the genus Aureob as idium showed different degrees of tolerance to different heavy metals, with MTCs for heavy metals ranging from 75 to 1,500 mg/L. The resistance test indicated that the AP strain exhibited a very high degree of resistance to almost all of the investigated heavy metals, and especially to Pb2+, to which it showed more than twice higher tolerance than to the others. It is well-known that heavy metals are toxic to living cells even at low concentration of about 1.0–10 mg/L, and some, such as Hg and Cd, are very toxic even at lower concentration of 0.001–0.1 mg/L40. The heavy metal tolerance test indicated maximum microbial tolerance of Pseudomonas sp. to Cu at 300 mg/L41, and Streptomyces sp. to Hg at 200 mg/L42, while 100 mg/L Cd was considered as the MTC of the metal for most of the fungus43. Furthermore, we have shown that the ability of cells to withstand the lethal effect of heavy metals rises with the increase in their trehalose content. The APT∆A double mutant strain was relatively more tolerant to most heavy metals under investigation, with MTC increases ranging from 20 to 60%. Overall, the pattern of metal tolerance was in the order of Pb2+ > Hg2+ > Cu2+ > Cd2+ > Co2+ > Ni2+ > Zn2+.
Figure 4.
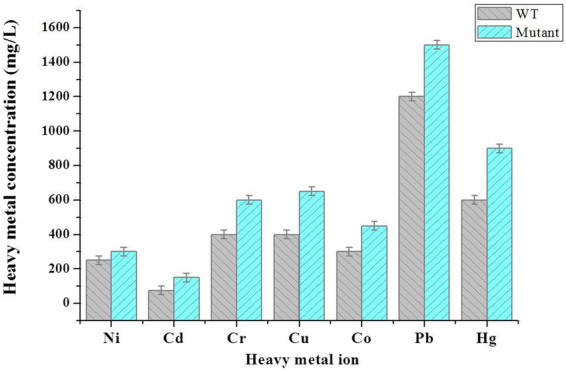
The resistance to different concentrations of heavy metal of isolates of Aureobasidium strain wild-type AP and modified mutant strain APT∆A. Each error bar indicates +/− one standard error of the mean.
In order to investigate the roles of relevant genes in the alteration of the cellular phenotype in relation to heavy metal stress tolerance between the wild-type AP and the mutant strain APT∆A, qRT-PCR was performed. Genes involved in DNA repair (e.g., rad1, rad25, mutL) and the partial oxidases, such as FeSOD, ccp1, caos and gsho1, displayed strikingly increased expression levels in the mutant strain APT∆A (>10-fold). Moreover, genes involved in oxidative stress (e.g., svf1, rci1) and transporter genes (e.g., ABC1, qutD) were up-regulated 2–4 fold when trehalose was overproduced in the mutant cells (Table 3). Trehalose is a stress-induced metabolite and has the ability to protect the cells as a whole, as well as the cell membranes, proteins and other biological macromolecules under various adverse environmental conditions35. In this study, the strongest evidence for the protective role of trehalose was that the mutant strain APT∆A with more trehalose accumulation had a markedly enhanced resistance to Pb2+. Most likely, trehalose enhances resistance to heavy metal stress by quenching cations generated either in the medium or inside cells after entry of metal ions (e.g., Pb2+), through the promotion of the expression of the above functional genes. This observation was consistent with a previous report on cells exposed to a free radical-generating system (H2O2/iron)36. Our previous study demonstrated that trehalose also improved the robustness of Propionibacterium acidipropionici, especially in response to acid stress44. However, this is, to the best of our knowledge, the first study to directly demonstrate that the ability of microorganisms to survive under heavy metal stress is related to the intracellular concentration of trehalose.
Table 3.
qRT-PCR results of genes related to Pb2+ tolerancea.
| Gene | Expression level (Avg. ± std) | Fold change (APT∆A/AP) | ||
|---|---|---|---|---|
| Abbr. | Full name of expression product | Wild-type AP | Mutant APT∆A | |
| rad25 | DNA repair helicase | 2.12 ± 0.45 | 38.48 ± 1.97 | 18.15 |
| rad1 | DNA repair exonuclease | 3.66 ± 1.01 | 62.66 ± 3.32 | 17.12 |
| mutL | DNA mismatch repair protein | 1.98 ± 0.87 | 30.43 ± 1.56 | 15.37 |
| svf1 | Oxidative stress survival protein | 13.28 ± 1.64 | 29.61 ± 1.86 | 2.23 |
| rci1 | Oxidative stress response RCI peptide | 17.83 ± 0.89 | 56.88 ± 2.99 | 3.19 |
| ABC1 | ATP-binding cassette transporter | 21.01 ± 1.67 | 60.51 ± 3.23 | 2.88 |
| qutD | Major facilitator superfamily (MFS) transporter | 10.16 ± 2.18 | 37.90 ± 2.37 | 3.73 |
| FeSOD | Iron superoxide dismutase | 4.11 ± 1.28 | 47.51 ± 2.41 | 11.56 |
| ccp1 | Cytochrome c peroxidase | 2.09 ± 0.33 | 22.95 ± 1.24 | 10.98 |
| caos | Copper-containing amine oxidases | 1.55 ± 0.19 | 18.80 ± 1.12 | 12.13 |
| gsho1 | Glutathione peroxidase | 3.39 ± 1.16 | 54.69 ± 2.74 | 16.13 |
aThe concentration of lead ion (Pb2+) was 0.10% (1,000 mg/L). All data are obtained as the mean of three tests with P < 0.05.
Conclusion
Samples from soils polluted by radiation and heavy metals were collected in Xinjiang, Uyghur Autonomous Region of Northwest China, and a novel isolate was selected on enrichment media containing Pb2+. The phylogenetic tree based on the LSU rRNA gene suggested that this isolate should be classified as a novel strain of Aureobasidium subglaciale and was therefore designated as A. subglaciale F134. In addition, the trehalose content of strain F134 has been manipulated through the overexpression of the trehalose-6-phosphate synthase gene tps1 and deletion of the acid trehalase gene ath1. Compared with the wild-type AP strain, the mutant strain APT∆A had a 3-fold increased trehalose content, which significantly improved both its radiation- and its heavy-metal tolerance. Further investigation of the relevant genes related to heavy metal stress tolerance showed that genes encoding DNA repair proteins, oxidases, oxidative stress response factors, and transporter proteins were up-regulated dramatically when trehalose was overproduced in the mutant cells. This study is the first to report on the protective effects of trehalose against radiation and heavy metal stress in the genus Aureobasidium, and expands our knowledge of the diversity of radiation and heavy metal-resistant microorganisms.
Materials and Methods
Sampling sites and sample collection
Samples were taken from Chinese nuclear radiation polluted areas located in the Xinjiang Uyghur Autonomous Region of Northwest China. The samples were collected in October 2011 from the middle level of pollution soils (1,000–2,000 Bq/kg)45 at a depth of approximately 5–20 cm, and were immediately preserved in sterile centrifuge tubes (50 mL) at 4 °C, and transported to the laboratory in dark conditions.
Selective media and isolation
Approximately 2.0 g of each sample was added to the liquid enrichment media as inoculum. The enrichment media were as follows: Czapek medium, PDA medium, MEA medium, and YPD medium. All media were supplemented with chloramphenicol (50 mg/L) to selectively inhibit the growth of bacteria.
The soil samples were filtered through a 2 mm pore size mesh, and approximately 1–2 g portions were individually transferred into 15 × 150 mm aseptic tubes with 5 mL of sterile Czapek liquid medium and cultivated under shaking at 28 °C for 3 days. The resulting enrichment cultures were diluted 10, 20 and 30-fold, and 0.02% of the dilution was spread on plates comprising Czapek or PDA agar, both with 0.05% Pb2+, and incubated at 28 °C for 7 days. Colony growth was observed daily, and single colonies were selected based on colony characteristics, transferred to fresh agar plates for further purification and stored on PDA agar slants at 4 °C for later identification and characterization.
Molecular identification
Fresh yeast cells were transferred into MEA broth and cultured under shaking at 28 °C for 60 h. The cells were collected by centrifugation and resuspended in TE buffer 3 times, after which the genomic DNA was extracted using the E.Z.N.A.TM Yeast DNA Spin Protocol Kit (Omega Bio-Tek, Georgia, USA) according to the manufacturer’s instructions, and stored at −20 °C for further study. The sequences of the ITS region and the D1/D2 region of the LSU rRNA gene were amplified from the extracted A. pullulans genomic DNA using the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′), ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), and the primers NL-1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′), NL-4 (5′-GTCCGTGTTTCAAGACGG-3′), respectively. The polymerase chain reaction (PCR) was carried out using the following amplification conditions: 95 °C for 5 min, followed by 35 cycles comprising 94 °C for 1 min, 52 °C for 1 min and 72 °C for 2 min, followed by a final elongation step at 72 °C for 10 min. Before sequencing, the amplified PCR products were purified using the Axy PrepTM DNA Gel Extraction Kit (Qiagen, Chatsworth, CA), and the concentrations of the purified products estimated based on band intensity following agarose gel electrophoresis. DNA was sequenced using the ABI3730 sequencer (Applied Biosystems, Foster City, CA) with the indicated primers, to obtain a result permitting an identification of the taxonomic status of the strain.
The sequence data was integrated from bidirectional sequencing using software ContigExpress (Vector NTI Suite 6.0, InforMax Inc.). BLAST was used to pairwise compare the obtained sequences with information of related species from the GenBank. MEGA (version 7.0) was used to reconstruct a phylogenetic tree and conduct cluster analysis by using the neighbor-joining method, and ascertain the biological classification status of the isolated strain. The D1/D2 domain of the LSU rRNA gene was aligned automatically using ClustalW. The generated phylogeny was tested using the bootstrap method set at 1000 iterations. Bootstrap analysis was used to estimate the confidence levels of the clades, and values that were greater than 50% were displayed on the phylogenetic tree.
Genetic manipulation of the trehalose biosynthesis pathway
To overexpress the TPS1 gene, the corresponding DNA fragment was amplified by PCR from the chromosomal DNA of A. pullulans and ligated into the KpnI-ApaI digested plasmid pUG6E, containing the kanMX resistance marker. The TPS1-overexpression strain was obtained through the homologous recombination of the StuI-linearized plasmid pUG6E-TPS1 and the TPS1 gene in the genome. The transformants were selected on YPD plates containing 300 μg/mL G418. The engineered haploid was named APtps1 (MAT α, PGK-TPS1-kanMX).
Gene disruption using the disruption cassette was performed based on the method by Güldener et al.46. The ath1 deletion strain was obtained by a one-step disruption of ATH1. The ATH1 disruption cassette contained, from left to right, the ATH1A fragment comprising the 500 bp upstream of the ATG start codon of ATH1, the ble gene, and the ATH1B fragment comprising the 500 bp downstream of ATH1. The strains AP and APtps1 were transformed with the ATH1 deletion cassette, and the ath1 knockout strains were selected on YPD medium containing 50 μg/mL zeocin. The ath1 knockout strain was named AP∆ath1 (MAT α, ∆ath1::ble), and the mutant strain with tps1 overexpression and ath1 deletion was named APT∆A (MAT α, PGK-TPS1-kanMX, ∆ath1::ble).
The A. subglaciale strains were transformed using the LiAc/single-stranded carrier DNA/PEG method47. Proper integration was verified via diagnostic PCR and sequence analysis.
Radiation resistance analysis
Cell suspensions (OD600 ~1.0) were divided into 2 mL aliquots and exposed to a gamma source at a dose rate of 100 Gy/min at room temperature; the delivered gamma radiation doses spanned from 0 to 15.0 kGy in steps of 2.5 kGy. The thus treated samples were spread directly or after suitable serial dilution onto MEA agar plates and incubated in the dark at 28 °C for 10 days to count the colony-forming units.
UV irradiation experiments were performed using a 15 W UV-B lamp. Cell suspensions (OD600 ~1.0) were divided into 10 mL aliquots and placed into 9 cm diameter plates. The plates were exposed to UV light at a distance of 30 cm. The time of UV irradiation was from zero to 15 min in steps of 2.5 min. The treated samples were plated directly or after suitable dilution onto MEA agar plates and incubated at 28 °C for 10 days to count the colony-forming units.
Viability was assessed by using non-irradiated suspensions of each strain under the same conditions as the controls. E. coli was used as a negative control in both gamma and UV irradiation experiments, with D. radiodurans R1 as a positive control.
Maximum Tolerance Concentrations (MTC) of heavy metals
To determine the MTC of the strain for different heavy metals, Czapek medium incorporated with different concentrations of Ni2+, Hg2+, Cu2+, Co2+, Cd2+, Pb2+, and Cr2+ was used. The concentration range of all heavy metal ions was from 50 mg/L to 1,500 mg/L. The cells were grown under optimal culture conditions at 28 °C for 10 days48.
Analytical methods
Cell growth was analyzed by measuring the optical density of the cell suspensions at 600 nm (OD600) on an Ultrospec 3300 pro spectrophotometer (Amersham Bioscience, United Kingdom) after appropriate dilution. Cell morphology was analyzed by direct observation under an Olympus CX23 microscope (Olympus, Japan) and a Zeiss Libra 200 transmission electron microscope (Carl Zeiss AG, Germany). The concentration of trehalose in the cell extracts was determined by the enzymatic trehalase method (Sigma-Aldrich, USA) as described previously44. Samples for dry weight analysis were washed with distilled water and dried at 105 °C for 12 h. TPS and ATH activities were determined as reported previously49. Quantitative Real-time Reverse Transcription PCR (qRT-PCR) was carried out to verify the RNA-seq results using an ABI STEPONE PLUS Real Time PCR System50. Primer Premier 6.0 (PREMIER Biosoft International, Palo Alto, CA, USA) was used to design primers for the target genes (Table 4). All results are based on triplicate determinations.
Table 4.
qRT-PCR primers used in this study.
| Gene | Primer | Sequence (5′ → 3′) |
|---|---|---|
| rad25 | Forward | TCACAAATCCGCTTTCGAAGCCGCT |
| Reserve | ATGCCGCCCAAGAGGAAAGCCCCTG | |
| rad1 | Forward | ATGGCCCCAACAAACACATGTCTA |
| Reserve | TCACTCGTCGTCCGTCTCTGAATCA | |
| mutL | Forward | ATGGAAGAGAACGGAGATGTGGAG |
| Reserve | TCAACATCTTTCAAACACGCGGTAC | |
| svf1 | Forward | ATGATGAACTGGGCGAAACAACAGC |
| Reserve | TCATGAGATGAAAGTGGCCTCAGTG | |
| rci1 | Forward | ATGTGCGGCAGCGATATCTTCTTGG |
| Reserve | CTAGTCGTGTGACTGGACCTTGTTG | |
| ABC1 | Forward | CTAGATGACTGTAGGTTGCATGCCC |
| Reserve | ATGAAAGGAACGTTTGGATGGGCTC | |
| qutD | Forward | ATGGGTGTCCTTGCCAAAATCGAAG |
| Reserve | CTACGCCCTATGCTCTGAAGACTGA | |
| FeSOD | Forward | ATGGCCGTCACACAATACTCACTAC |
| Reserve | TCAAATGCTCGCACGAAGGAATTTA | |
| ccp1 | Forward | TTACTCGGAAGGCTTGAAGGTCATG |
| Reserve | ATGGCTTCCGCTGCCAGATCTTTCA | |
| caos | Forward | ATGGCTCCCTCTCATCCTCTTTCCA |
| Reserve | TTAGAGCTTTCCCTGAGCAGGTTG | |
| gsho1 | Forward | ATGTCTTCTGCTACCTCTTTCCACGA |
| Reserve | TTAAGCCTTGAGCTGAGCCTCGATG |
Electronic supplementary material
Acknowledgements
This work was supported by the National Science Foundation of China (21506101, 31560034, U1603112, 2016D01A050), the State Key Laboratory of Bio-organic and Natural Products Chemistry, CAS (SKLBNPC15429), the Six Talent Peaks Project in Jiangsu Province (2015-JY-009), and the Environmental Protection Project in Jiangsu Province (2015053).
Author Contributions
T.T.L. performed the experiments, L.Y.Z. collected and analyzed the data, Z.D.Z. rafted the manuscript; Z.P.Z. assisted in conceiving and designing the experiments, L.J. revised the manuscript; and H.H. contributed reagents, materials and analytical tools. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15489-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zhidong Zhang, Email: zhangzheedong@sohu.com.
Ling Jiang, Email: jiangling@njtech.edu.cn.
References
- 1.Brim H, McFarlan SC, Fredrickson JK, Minton KW, Zhai M. Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat. Biotechnol. 2000;18:85–90. doi: 10.1038/71986. [DOI] [PubMed] [Google Scholar]
- 2.Wu Q, Zhu L, Jiang L, Xu X, Xu Q. Genome sequence of Paenibacillus wulumuqiensis sp. nov., a bioflocculant-producing species. Genome Announc. 2015;3:e00795–00715. doi: 10.1128/genomeA.00795-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Q, Zhu L, Jiang L, Xu X, Xu Q. Draft genome sequence of Paenibacillus dauci sp. nov., a carrot-associated endophytic actinobacteria. Genomics. Data. 2015;5:241–253. doi: 10.1016/j.gdata.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu L, Wu Q, Xu Q, Xu X, Jiang L. Draft genome sequence of Paenibacillus algorifonticola sp. nov., an antimicrobial-producing strain. Genomics. Data. 2015;5:302–308. doi: 10.1016/j.gdata.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang L, Lin M, Li X, Cui H, Xu X. Genome sequence of Thermus thermophilus ATCC 33923, a thermostable trehaloseproducing strain. Genome Announc. 2013;1:e00493–00413. doi: 10.1128/genomeA.00493-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, Jiang L, Zhang Z, Shi Y, Huang H. Genome sequence of a gamma-and UV-ray-resistant strain, Deinococcus wulumuqiensis R12. Genome Announc. 2013;1:e00206–00213. doi: 10.1128/genomeA.00206-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Y, Xu X, Song P, Jiang L, Zhang Z. Draft genome sequence of Deinococcus xibeiensis R13, a new carotenoid-producing strain. Genome Announc. 2013;1:e00987–00913. doi: 10.1128/genomeA.00987-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhdanova NN, Zakharchenko VA, Vember VV, Nakonechnaya LT. Fungi from Chernobyl: mycobiota of the inner regions of the containment structures of the damaged nuclear reactor. Mycol. Res. 2000;104:1421–1426. doi: 10.1017/S0953756200002756. [DOI] [Google Scholar]
- 9.Zhdanova NN, Tugay T, Dighton J, Zheltonozhsky V, Mcdermott P. Ionizing radiation attracts soil fungi. Mycol. Res. 2004;108:1089–1096. doi: 10.1017/S0953756204000966. [DOI] [PubMed] [Google Scholar]
- 10.Gu M, Zhang Z, Wang W, Tang Q, Song S. The effects of radiation pollution on the population diversities and metabolic characteristics of soil microorganisms. Water Air Soil Poll. 2014;225:2133–2140. doi: 10.1007/s11270-014-2133-4. [DOI] [Google Scholar]
- 11.Elbein AD, Pan Y, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. 2003;13:17R–27R. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Lin M, Xu X, Li S, Huang H. Identification and characterization of a novel trehalose synthase gene derived from saline-alkali soil metagenomes. PloS One. 2013;8(10):e77437. doi: 10.1371/journal.pone.0077437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avonce N, Mendoza VA, Morett E, Iturriaga G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006;6:109–114. doi: 10.1186/1471-2148-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoog GS. Evolution of black yeasts: possible adaptation to the human host. Anton. Leeuw. Int. J. G. 1993;63(2):105–109. doi: 10.1007/BF00872386. [DOI] [PubMed] [Google Scholar]
- 15.Gostinčar C, Ohm RA, Kogej T, Sonjak S, Turk M. Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genomics. 2014;15:549–576. doi: 10.1186/1471-2164-15-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson SW, Manitchotpisit P. & Leathers, T. D. Aureobasidium thailandense sp. nov. isolated from leaves and wooden surfaces. Int. J. Syst. Evol. Micr. 2013;63:790–795. doi: 10.1099/ijs.0.047613-0. [DOI] [PubMed] [Google Scholar]
- 17.Cimerman NG, Cimerman N, Zalar P, Hoog S, Plemenitaš A. Hypersaline waters in salterns-natural ecological niches for halophilic black yeasts. FEMS Microbiol. Ecol. 2000;32:235–240. doi: 10.1111/j.1574-6941.2000.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 18.Urzì C, Leo FD, Passo CL, Criseo G. Intra-specific diversity of Aureobasidium pullulans strains isolated from rocks and other habitats assessed by physiological methods and by random amplified polymorphic DNA (RAPD) J. Microbiol. Meth. 1999;36:95–105. doi: 10.1016/S0167-7012(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 19.Punnapayak H, Sudhadham M, Prasongsuk S, Pichayangkura S. Characterization of Aureobasidium pullulans isolated from airborne spores in Thailand. J. Ind. Microbiol. Biot. 2003;30:89–94. doi: 10.1007/s10295-002-0016-y. [DOI] [PubMed] [Google Scholar]
- 20.Sasahara H, Izumori K. Production of L-sorbitol from L-fructose by Aureobasidium pullulans LP23 isolated from soy sauce mash. J. Biosci. Bioeng. 2005;100:223–226. doi: 10.1263/jbb.100.223. [DOI] [PubMed] [Google Scholar]
- 21.Jiang H, et al. Both a PKS and a PPTase are involved in melanin biosynthesis and regulation of Aureobasidium melanogenum XJ5-1 isolated from the Taklimakan desert. Gene. 2017;602:8–15. doi: 10.1016/j.gene.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Santos VL, Monteiro AS, Braga DT, Santoro MM. Phenol degradation by Aureobasidium pullulans FE13 isolated from industrial effluents. J. Hazard. Mater. 2009;161:1413–1420. doi: 10.1016/j.jhazmat.2008.04.112. [DOI] [PubMed] [Google Scholar]
- 23.Zemek J, Ježo I. Induction by α, ά-trehalose derivatives of α, ά trehalase from Saccharomyces cerevisiae and Aureobasidium pullulans. Folia Microbiol. 1987;32(5):417–420. doi: 10.1007/BF02887572. [DOI] [Google Scholar]
- 24.Managbanag JR, Torzilli AP. An analysis of trehalose, glycerol, and mannitol accumulation during heat and salt stress in a salt marsh isolate of Aureobasidium pullulans. Mycologia. 2002;94:384–391. doi: 10.1080/15572536.2003.11833203. [DOI] [PubMed] [Google Scholar]
- 25.Iskandarov US, Guzalova AG, Davranov KD. Effects of nutrient medium composition and temperature on the germination of conidia and the entomopathogenic activity of the fungi Beauveria bassiana and Metarhizium anisopliae. Appl. Biochem. Micro. 2006;42:72–76. doi: 10.1134/S000368380601011X. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Hall C, Wolf-Hall C, Manthey F. Fungistatic activity of flaxseed in potato dextrose agar and a fresh noodle system. Int. J. Food Microbiol. 2008;121:262–267. doi: 10.1016/j.ijfoodmicro.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Ravella SR, Quiñones TS, Retter A, Amon T, Hobbs PJ. Extracellular polysaccharide (EPS) production by a novel strain of yeast-like fungus Aureobasidium pullulans. Carbohyd. Poly. 2010;82(3):728–732. doi: 10.1016/j.carbpol.2010.05.039. [DOI] [Google Scholar]
- 28.Bandara A, Fraser S, Chambers PJ, Stanley GA. Trehalose promotes the survival of Saccharomyces cerevisiae during lethal ethanol stress, but does not influence growth under sublethal ethanol stress. FEMS Yeast Res. 2009;9:1208–1216. doi: 10.1111/j.1567-1364.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahmud SA, Nagahisa K, Hirasawa T, Yoshikawa K, Ashitani K. Effect of trehalose accumulation on response to saline stress in Saccharomyces cerevisiae. Yeast. 2009;26:17–30. doi: 10.1002/yea.1646. [DOI] [PubMed] [Google Scholar]
- 30.Chan GF, Bamadhaj HM, Gan HM, Rashid NAA. Genome sequence of Aureobasidium pullulans AY4, an emerging opportunistic fungal pathogen with diverse biotechnological potential. Eukaryot. Cell. 2012;11:1419–1420. doi: 10.1128/EC.00245-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gostinčar C, Muggia L, Grube M. Polyextremotolerant black fungi: oligotrophism, adaptive potential, and a link to lichen symbioses. Front. Microbiol. 2012;3:390–395. doi: 10.3389/fmicb.2012.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conlin LK, Nelson HC. The natural osmolyte trehalose is a positive regulator of the heat-induced activity of yeast heat shock transcription factor. Mol. Cell. Biol. 2007;27:1505–1515. doi: 10.1128/MCB.01158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kandror O, DeLeon A, Goldberg AL. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA. 2002;99:9727–9732. doi: 10.1073/pnas.142314099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin Y, Zhang M, Adhikari B. Effect of trehalose and ultrasound-assisted osmotic dehydration on the state of water and glass transition temperature of broccoli (Brassica oleracea L. var. botrytis L.) J. Food Eng. 2013;119:640–647. doi: 10.1016/j.jfoodeng.2013.06.035. [DOI] [Google Scholar]
- 35.Tapia H, Young L, Fox D, Bertozzi CR, Koshland D. Increasing intracellular trehalose is sufficient to confer desiccation tolerance to Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2015;112:6122–6127. doi: 10.1073/pnas.1506415112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benaroudj N, Lee DH, Goldberg AL. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol.Chem. 2001;276:24261–24267. doi: 10.1074/jbc.M101487200. [DOI] [PubMed] [Google Scholar]
- 37.Sun N, Lee S, Song KB. Characterization of a carotenoid-hyperproducing yeast mutant isolated by low-dose gamma irradiation. Int. J. Food Microbiol. 2004;94:263–267. doi: 10.1016/S0168-1605(03)00311-8. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman PD, Kobayashi R, Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Gene Dev. 1997;11(3):345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Chen C. Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol. Adv. 2006;24(5):427–451. doi: 10.1016/j.biotechadv.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Singh V, Chauhan PK, Kanta R, Dhewa T, Kumar V. Isolation and characterization of Pseudomonas resistant to heavy metals contaminants. Int. J. Pharma. Sci. Rev. Res. 2010;3:164–167. [Google Scholar]
- 41.Sanjenbam, P., Saurav, K. & Kannabiran, K. Biosorption of mercury and lead by aqueous Streptomyces VITSVK9 sp. isolated from marine sediments from the bay of Bengal, India. Front. Chem. Sci. Eng. 1–5 (2012).
- 42.Pattanayak B, Mittra B, Dhal NK. Role of Morphology, Development and characterization of Cd accumulating mutants of Aspergillus nidulans. Int. J. Curr. Microbiol. App. Sci. 2015;4(5):695–710. [Google Scholar]
- 43.White O, Eisen JA, Heidelberg JF, Hickey EK, Peterson JD. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang L, Cui H, Zhu L, Hu Y, Xu X. Enhanced propionic acid production from whey lactose with immobilized Propionibacterium acidipropionici and the role of trehalose synthesis in acid tolerance. Green Chem. 2015;17:250–259. doi: 10.1039/C4GC01256A. [DOI] [Google Scholar]
- 45.Gu M, et al. The effects of radiation pollution on the population diversities and metabolic characteristics of soil microorganisms. Water Air Soil Poll. 2014;225(9):2133–2140. doi: 10.1007/s11270-014-2133-4. [DOI] [Google Scholar]
- 46.Güldener U, Heck S, Fiedler T, Beinhauer J, Hegemann JH. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic. Acids Res. 1996;24:2519–2524. doi: 10.1093/nar/24.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson BR, Lawrence SJ, Leclaire JP, Powell CD, Smart KA. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 2007;31:535–569. doi: 10.1111/j.1574-6976.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 48.Azabou S, Mechichi T, Patel BK, Sayadi S. Isolation and characterization of a mesophilic heavy-metals-tolerant sulfate-reducing bacterium Desulfomicrobium sp. from an enrichment culture using phosphogypsum as a sulfate source. J. Hazard. Mater. 2007;140:264–270. doi: 10.1016/j.jhazmat.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 49.Wang PM, Zheng DQ, Chi XQ, Li O, Qian CD. Relationship of trehalose accumulation with ethanol fermentation in industrial Saccharomyces cerevisiae yeast strains. Bioresource Technol. 2014;152:371–376. doi: 10.1016/j.biortech.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, Jiang L, Zhu L, Xu Q, Xu X. Tailoring of global transcription sigma D factor by random mutagenesis to improve Escherichia coli tolerance towards low-pHs. J. Biotechnol. 2016;224:55–63. doi: 10.1016/j.jbiotec.2016.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.